FDA also told us its expectations for additional data on COVID vaccines after the EUAs were issued
According to FDA criteria, the EUAs should have been revoked once the real world data rolled in
I have some more of FDA's power points from December 10, 2020 for you.
https://www.fda.gov/media/144329/download
Last week, I laid out FDA's criteria for issuing an EUA here, and showed that (at a minimum for the pediatric age groups), the Pfizer vaccine failed to meet at least 5 of those criteria. How could the vaccine have gotten EUAs? Good question.
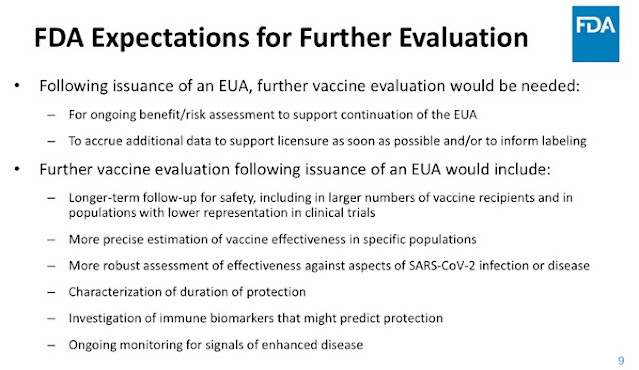
But FDA's pre-authorization requirements weren't all that FDA's Doran Fink, MD, PhD was there to talk about. He also presented information on the expectations FDA had after issuing an EUA, given that not a lot of data were required initially.
Below is what FDA said would be needed for its ongoing assessment. This makes sense. Did FDA take the required information into consideration? What information has it relied on?? Or has it, perhaps, relied on fake information, such as that generated by the Centers for Propaganda in Atlanta? Can we demand to see what FDA has relied on for its continuing assessments regarding the benefits of COVID vaccines?
* There needs to be ongoing evaluation of the risk vs. benefit for the vaccine.
* There must be continued safety followup using real world data.
* You can't guess at the degree of effectiveness: more precise and robust assessments of vaccine effectiveness will be required.
* You will need to see how long protection lasts.
* You have to keep looking for ADE aka VED. What else?
* Both active and passive monitoring for adverse effects for continue.
* The double-blinded, placebo controlled studies should be continued for as long as is feasible.
* FDA says just because we gave you an EUA, that does not mean you have to unblind the subjects or offer vaccine to those who received placebo. OTOH, trial participants may choose to leave a trial for every reason.
I think FDA was being disingenuous with this last statement, both to its advisors and to the public. Because practically as soon as the vaccine was authorized, FDA gave Pfizer the go-ahead to unblind the trial and vaccinate everybody, despite its advisers recommending against doing so.
* EUAs can be revoked, if the circumstances justifying them no longer exist, or if the issuing criteria are no longer met.
* If there are issues with safety or efficacy, manufacturing quality or the way the disease spreads in the population has changed, the EUA can be revoked.
We know very well that the issuing criteria have no longer been met: the efficacy is awful after the first few months, safety is a disaster, and manufacturing quality, with various extraneous and uncharacterized particles and nanoparticles in the vaccines should all have resulted in the revocation of the EUAs.






It is weird that we still think the FDA is, or should be accountable, honest, transparent, and immune to corruption. At this point we would have to be brain-dead or brainwashed to deny that they work for BigPharma as captured regulators, and that they all either do, or will work directly for BigPharma after their “public service work.”
It's shocking that these violations have gone this long, and also shocking that there seems to be very little attention to it, even by non-sheeple media and the few seemingly honest and awake congress people such as Ron Johnson.
Thank you Dr Nass for exposing this... hopefully it will soon get the attention it deserves.