How much is a little $moneypox worth? I also dissect the CDC/NEJM scam supporting the Jynneos vaccine's efficacy
If you can circumvent lawsuits for injuries (since we already know it causes myocarditis) it is worth a lot more.
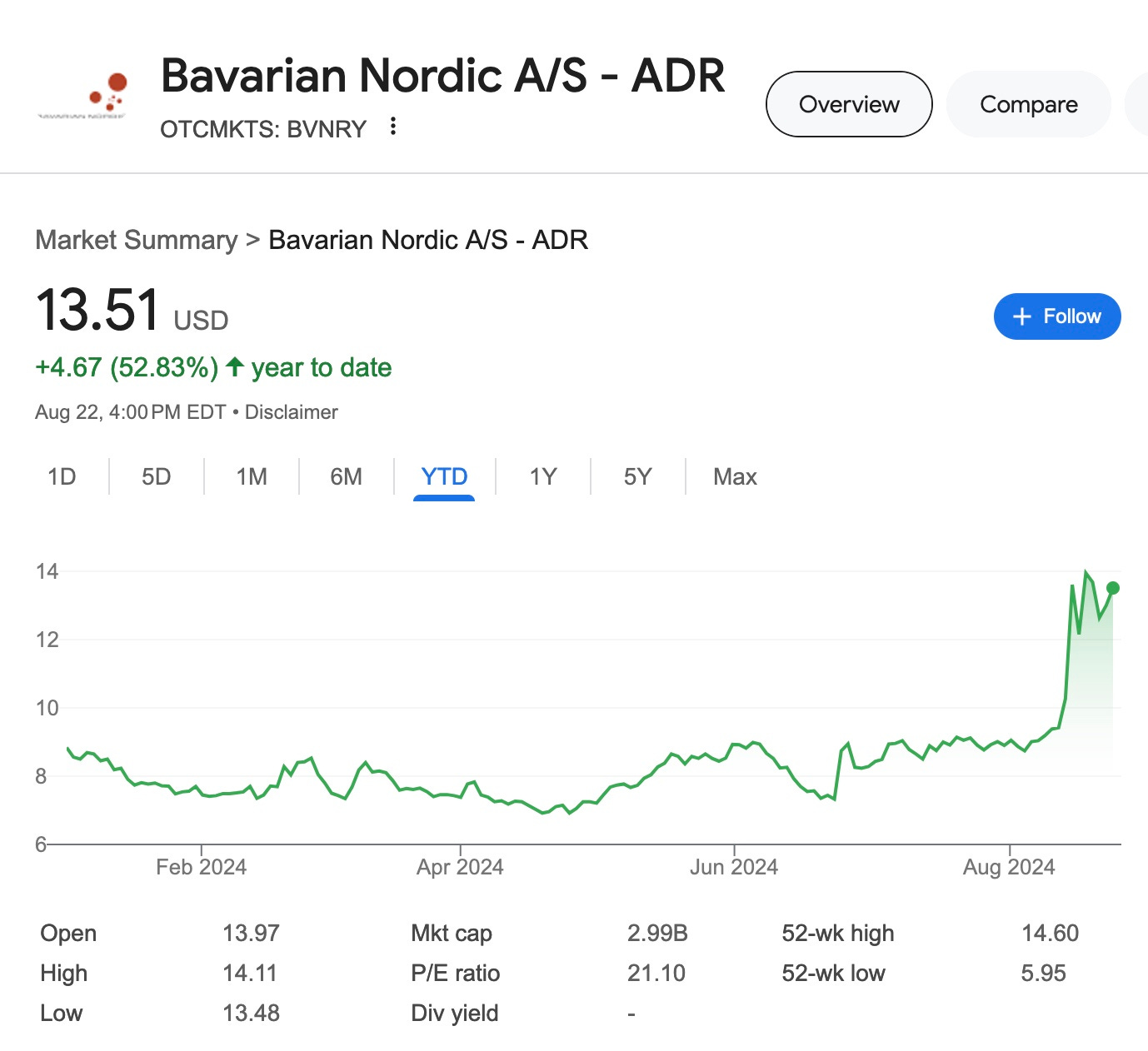
Here is Bavarian Nordic’s stock price year to date.
Tedros’ declaration naming Moneypock$ as a PHEIC increased Bavarian Nordic’s stock value by about $300 million dollars in an afternoon 8 days ago.
Bavarian Nordic is a small pharma company bound at the hip to BARDA. They make Jynneos vaccine, aka MVA-BN, aka Imvamune, aka Imvanex. It was fully licensed by FDA in September 2019 (as COVID was kicking off) as a vaccine to protect against smallpox and monkeypox. At the time of licensure, there existed no admitted evidence the vaccine could prevent either. The only so-called efficacy data were antibody levels, which imho are essentially worthless. Now, there was that CDC trial in the Congo of 1,000 healthcare workers I keep mentioning, but it is never mentioned officially; only the announcement that it was underway is available:
As far as CDC and FDA go, the safety and efficacy data from that trial evaporated.
Efficacy for Jynneos in 2024 should be very well known, since over 700,000 “high risk” recipients were vaccinated for monkeypox in 2022 and 2023 in the US alone, most with a 1/5 dose of Jynneos (intradermal injection), some with full strength Jynneos (subcutaneously administered) and some with ACAM2000 full dose. All the intradermal Jynneos and all the ACAM2000 were given under CICP-Prep Act with no liability for anyone involved.
I don’t know whether the government successfully found a way to turn Jynneos, a fully licensed vaccine, given for its labeled indication (monkeypox) at the labeled dose into a liability-free EUA product. According to the law you cannot turn a licensed drug, used correctly for its licensed indication, into an EUA. But the USG/FDA made it happen for some COVID shots anyway….
I am certain the government lawyers worked overtime to find a way to shield everyone from liability with the initial full strength doses of Jynneos… until someone figured out they could waive liabililty by giving the vaccine at a new reduced dose, using a new method of administration, and thereby turn it into an EUA product.
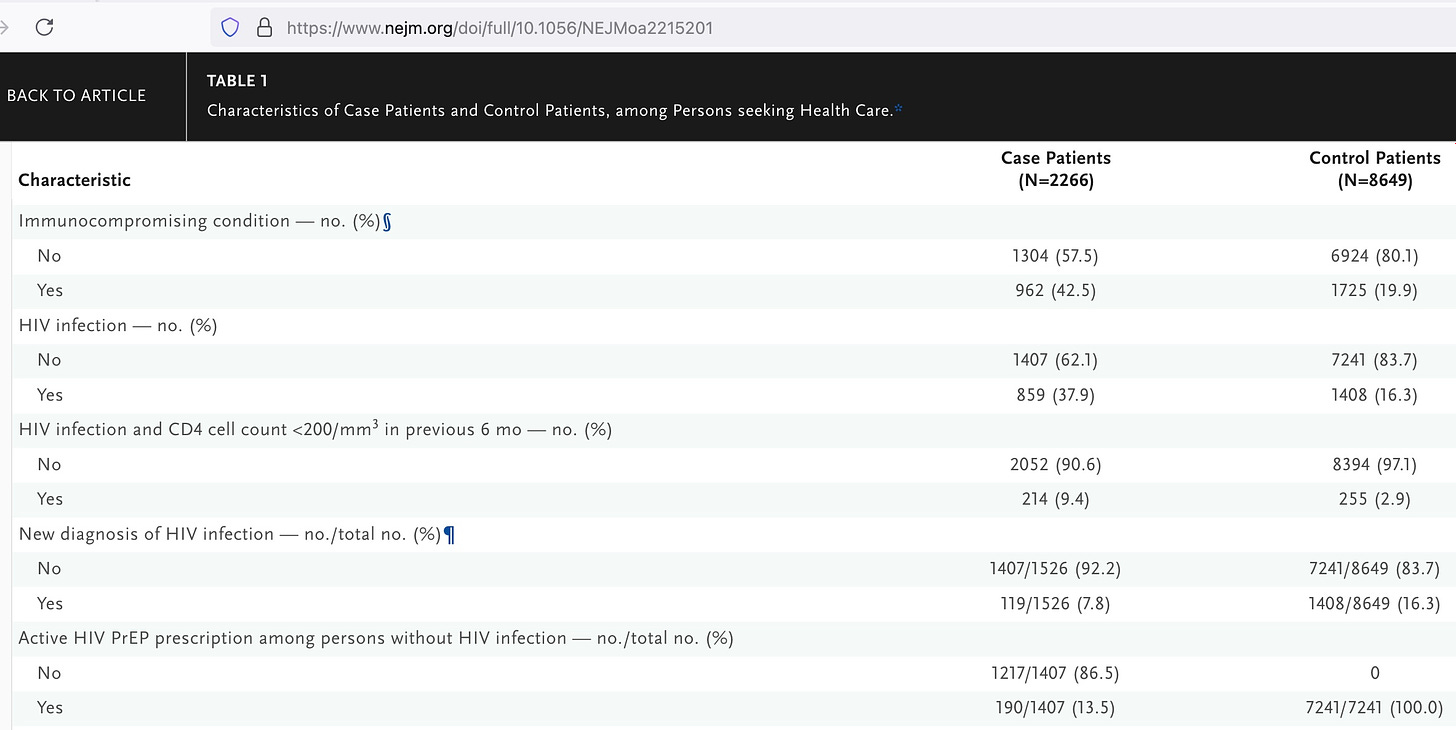
And yet while there should be good data on Jynneos, there is only limited, poor quality data and a bad study design to answer the question of whether and how well the vaccine actually works to prevent monkeypox. Here’s the major flaw I found, in addition to the small number of cases and the case-control method:
The controls all had HIV (about 20%) OR were taking drugs to prevent HIV (80%).
Only 13.5% of the HIV negative cases were taking medication to prevent HIV.
Presumably then, the control group considered itself at much higher risk for HIV than the cases, which would reflect a greater number of sexual partners. See how the CDC authors tried to fool us? They chose a “control” group that would be at much higher risk of sexually transmitted monkeypox, in order to make the vaccine appear more effective.
The study was designed by staff from the Centers for Disease Control and Prevention (CDC) and Epic Research; staff from Epic Research gathered and analyzed the data, and staff from the CDC and Epic Research vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol…
I’m sure CDC had data on 700,000 vaccine recipients but chose to use a commercial electronic medical record instead of its own more comprehensive data and a funky methodology to claim 66% efficacy for 2 doses of vaccine, with a wide confidence interval.
We categorized the route of administration as subcutaneous, intradermal, other (i.e., improbable administration routes, including intramuscular, oral, or sublingual), or missing. For vaccinations before August 9, 2022, we recategorized the route of administration from “other” or “missing” to “subcutaneous” because JYNNEOS was authorized only for subcutaneous administration during this time. For vaccinations on or after August 9, 2022, we used dosage information to recategorize the route of administration: vaccinations with a route documented as “other” or “missing” and a dose of 0.5 ml were recategorized as “subcutaneous,” and those with a dose of 0.1 ml were recategorized as “intradermal.” Vaccines administered on or after August 9, 2022, with a route of “missing” or “other” and a dose other than 0.1 ml or 0.5 ml were not recategorized.
Did I mention they lumped both doses together in their funky analysis, even though one was 5 times larger and the route of administration varied? Here is the excuse:
Limited sample sizes precluded the examination of vaccine effectiveness among persons who were fully vaccinated subcutaneously (69 patients) or intradermally (47 patients).
Here are the results:
After adjustment for age, race or ethnic group, SVI score, and the presence or absence of immunocompromising conditions, vaccine effectiveness was 35.8% (95% CI, 22.1 to 47.1) for partial vaccination and 66.0% (95% CI, 47.4 to 78.1) for full vaccination (Table 2). Among men 18 to 49 years of age who had not received ACAM2000 vaccination, adjusted JYNNEOS vaccine effectiveness was 35.5% (95% CI, 19.1 to 48.6) for partial vaccination and 58.7% (95% CI, 33.9 to 74.3) for full vaccination (Table 3). In patients without an immunocompromising condition, adjusted vaccine effectiveness was 40.8% (95% CI, 24.8 to 53.4) with partial vaccination and 76.3% (95% CI, 57.7 to 86.8) with full vaccination.
After claiming that a larger body of evidence supports Jynneos effectiveness, CDC acknowledged there were lots of caveats after all. Look at all the weasel words in the final paragraph:
This study, which used data from a national EHR data platform, suggested that JYNNEOS vaccine was effective at reducing the risk of mpox disease, with effectiveness that appeared to be greater among persons who completed the two-dose series. In addition to vaccination, persons might benefit from strategies to prevent or reduce the risk of acquiring or transmitting mpox.25,28 Further research is needed to understand vaccine effectiveness according to route of administration and among subpopulations at risk for severe outcomes.
And then there is this: nearly half the people who got one dose of Jynneos failed to return for the second dose. Wonder why?
Recent data suggest that only 57.6% of first-dose vaccine recipients who were eligible to receive a second dose did so.27




Create the fear, share price goes up, sell high, fear subsides, share price goes down, buy low, repeat.
Did anyone follow their (single dose recipients who never came back for dose two) outcomes?! Or were they dead?!