I will be live on Del Bigtee's The Highwire, Aug 31. In case you like watching in real time.
And 2 new documents for my myocarditis timeline--the FDA/CDC race against time to damage and kill kids for fun and profit
What did our public health agencies know, and when did they know it? And why did they carry on with the shots for children, despite knowing at the latest by end July 2021 that they did not prevent cases or transmission? Who called the shots?!!?
Feb. 28, 2021: Israel asks other public health agencies if they are also noticing high rates of myocarditis in teens and young adults after the shot. Since 80% of cases occurred within 4 days of the 2nd injection at that point, the link to vaccination was obvious. Feds had to know by then, if not earlier, to be looking carefully at the myocarditis data coming into their ~20 different databases, which were allegedly being scoured to identify vaccine adverse events..
April 25, 2021: The myocarditis story reached the wire services and was on TV in Israel. But most Americans were kept in the dark.
May 10, 2021: FDA authorizes the vaccine for adolescents despite knowing of the risk.
May 14, 2021: CDC claims in the MMWR that there are no specific safety signals. Quick, vaccinate those kiddies! Massachusetts repeats the claim.
May 24, 2021: ABC-TV reports 18 myocarditis cases in young people in Connecticut, a small state.
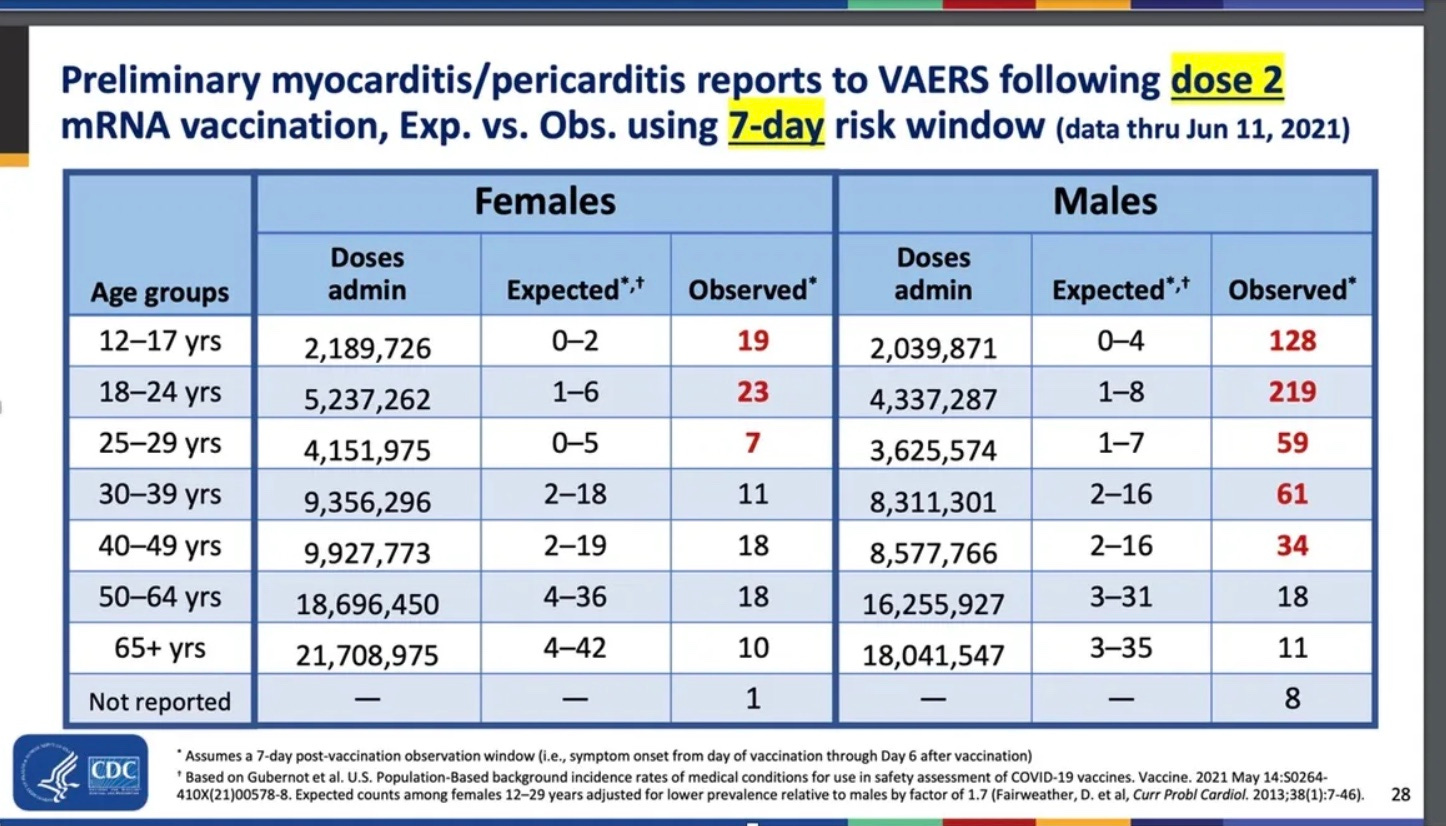
June 23, 2021: CDC’s Dr. Tom Shimabukuro finally found a signal for myocarditis in young people —he tells the audience at a CDC Advisory Committee on Immunization Practices (ACIP) meeting that the rate could be 100 times or more what would be expected from chance..
November 1992: But Ralph Baric could have told you his experimental coronaviruses caused myocarditis 30 years ago. Do you think he hid this from his very generous funders in DHHS? I doubt it…








Dear Dr. Nass, please note the Tromethamine cardio drug was added to the childhood vaccine version for compassionate slow kill purposes.
The Covid plandemic gene therapy vaccine is premeditated murder in the first degree.
1. The FDA Safety panel knew these shots were deadly right from the start. The Oct 22 2020 safety review featured a hugely damning slide 16 with its list of deadly symptoms. This product would NEVER have been approved for market were it not for the fact that the DoD and Barda were cramming the poison thru EUA at Warp Speed!
https://www.infowars.com/posts/oops-fda-accidentally-shows-list-of-covid-vaccine-side-effects-including-myocarditis-autoimmune-disease-death/
Vaccines and Related Biological Products Advisory Committee October 22, 2020 Meeting Presentation- COVID19 CBER Plans for Monitoring Vaccine Safety and Effectiveness *See Slide#16-17
https://www.fda.gov/media/143557/download
The slide is headlined, “FDA Safety Surveillance of COVID-19 Vaccines: DRAFT Working list of possible adverse event outcomes ***Subject to change***.”
2. Forgive this long damning evidence data dump. My spirit animal is a verbose Elephant that tries to never forget critical things:
Pfizer adds chemical Tromethamine to the child vaccines--a blood acid reducer used to stabilize people with heart attacks.
https://www.drugs.com/cdi/tromethamine.html
Latest Covid-19 vaccine side-effects -
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8062405/
And: https://www.sciencedirect.com/science/article/pii/S1323893021000459
Allergology International
Volume 70, Issue 3, July 2021, Pages 313-318
Review Article
Allergy to COVID-19 vaccines: A current update
They changed the formula in the kids vax to use tromomethamine
https://mobile.twitter.com/karenalainehunt/status/1455226904416374789
https://www.who.int/publications/m/item/comirnaty-covid-19-mrna-vaccine
https://presscalifornia.com/2021/10/28/pfizer-vax-kids/
For the Consumer
Applies to tromethamine: parenteral injection
Side effects include:
Adverse effects may include respiratory depression, local irritation, tissue inflammation, injection site infection, febrile response, chemical phlebitis, venospasm, hypervolemia, IV thrombosis, extravasation (with possible necrosis and sloughing of tissues), transient decreases in blood glucose concentrations, hypoglycemia, and hepatocellular necrosis with infusion via low-lying umbilical venous catheters. (See Warnings under Cautions.)
See page 14 near the bottom:
https://www.fda.gov/media/153447/download
12-15 years of age, and 5,821 U.S. reports were in adolescents 16-17 years of age. The top ten
most frequently reported MedDRA preferred terms (PTs) included:
• Overall most frequent PTs: headache, fatigue, pyrexia, SARS-CoV-2 test, dizziness,
pain, nausea, chills, pain in extremity, dyspnoea
• Most frequent PTs in in persons ≤17 years of age: dizziness, syncope, headache,
pyrexia, nausea, product administered to patient of inappropriate age, chest pain,
fatigue, vomiting, loss of consciousness.
Note that a report may have one or more PTs. An additional query of VAERS for U.S. reports by
dose number retrieved the following: 127,747 reports after Dose 1; 100,730 reports after Dose
2; and 5,223 reports after dose 3 (data as of October 18, 2021).
Safety concerns identified from post-authorization safety surveillance data in VAERS are
summarized below. Anaphylaxis, myocarditis, and pericarditis are existing safety concerns that
have been added to the product Fact Sheets. Review of passive surveillance AE reports and the
Sponsor’s periodic safety reports does not indicate any new safety concerns, including in
adolescents. Most AEs are labeled events and consistent with the safety profile for this vaccine.
No unusual frequency, clusters, or other trends for AEs were identified that would suggest a
new safety concern.
Anaphylaxis
Post-authorization surveillance has identified a risk of anaphylaxis, occurring at a rate similar to
reported rates of anaphylaxis following licensed preventive vaccines, primarily in individuals with
history of prior severe allergic reactions to other medications or foods.4243 Anaphylaxis is an
important identified risk in the pharmacovigilance plan (PVP) and included in the Warnings
sections of the vaccine Fact Sheets and Prescribing Information. The estimated crude reporting
rate for anaphylaxis in the U.S. is 6.1 cases per million doses at this time based on the above
VAERS data.
Myocarditis and pericarditis
Post-EUA safety surveillance reports received by FDA and CDC identified increased risks of
myocarditis and pericarditis, particularly within 7 days following administration of the second
dose of the 2-dose primary series. Reporting rates for medical chart-confirmed myocarditis and
pericarditis in VAERS have been higher among males under 40 years of age than among
females and older males and have been highest in males 12 through 17 years of age (~71.5
cases per million second primary series doses among males age 16-17 years and 42.6 cases
per million second primary series doses among males age 12-15 years as per CDC
presentation to the ACIP on August 30, 2021). In an FDA analysis of the Optum healthcare
claims database, the estimated excess risk of myocarditis/pericarditis approached 200 cases
per million fully vaccinated males 16-17 years of age and 180 cases per million fully vaccinated
males 12-15 years of age.44 Although some cases of vaccine-associated myocarditis/pericarditis
have required intensive care support, available data from short-term follow-up suggest that most
individuals have had resolution of symptoms with conservative management. Information is not
yet available about potential long-term sequelae and outcomes in affected individuals, or
whether the vaccine might be associated initially with subclinical myocarditis (and if so, what are
the long-term sequelae). A mechanism of action by which the vaccine could cause myocarditis
and pericarditis has not been established. Myocarditis and pericarditis were added as important
identified risks in the PVP and included in the Warnings sections of the vaccine Fact Sheets and
Prescribing Information. The Sponsor is conducting additional post-authorization/post-marketing
studies to assess known serious risks of myocarditis and pericarditis as well as to identify an
unexpected serious risk of subclinical myocarditis.
5 EUA AMENDMENT REQUEST FOR THE PFIZER-BIONTECH COVID-19 VACCINE FOR
USE IN CHILDREN 5-11 YEARS OF AGE
On October 6, 2021, Pfizer and BioNTech submitted a request to amend this EUA to include
use of a 2-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine (10 µg each dose,
administered 3 weeks apart) in individuals 5-11 years of age for active immunization to prevent
COVID-19 caused by severe acute coronavirus 2 (SARS-CoV-2).
The request is accompanied by safety data from 1,518 BNT162b2 and 750 placebo (saline)
Phase 2/3 participants 5-11 years of age in ongoing clinical study, C4591007, of which a total of
1,444 (95.1%) had safety follow-up ≥2 months after Dose 2 at the time of a September 6, 2021
data cutoff, and data from an additional 1,591 BNT162b2 and 788 placebo participants with a
median duration of follow-up of 2.4 weeks post-Dose 2 at the time of an October 8, 2021 data
cutoff. Vaccine effectiveness in children 5-11 years of age was inferred by immunobridging
SARS-CoV-2 50% neutralizing antibody titers (NT50, as assessed by SARS-CoV-2 mNG
microneutralization assay) among C4591007 study participants 5-11 years of age following
completion of a primary series to antibody titers of those of young adults 16-25 years of age
who received two doses of 30 μg BNT162b2 in study C4591001. Efficacy against COVID-19
disease was assessed descriptively in study C4591007 participants 5-11 years of age.
Vaccine formulation
Authorization is being requested for a modified formulation of the Pfizer‑BioNTech COVID-19
Vaccine. Each dose of this formulation contains 10 μg of a nucleoside-modified messenger RNA
(mRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2 that is formulated in lipid
particles and supplied as a frozen suspension in multiple dose vials.
To provide a vaccine with an improved stability profile, the Pfizer-BioNTech COVID-19 Vaccine
for use in children 5-11 years of age uses tromethamine (Tris) buffer instead of the phosphatebuffered
saline (PBS) as used in the previous formulation and excludes sodium chloride and
potassium chloride. The packaged vials for the new formulation are stored frozen at -90°C to -
60°C. The frozen vials may be thawed and stored at refrigerator at 2°C to 8°C for up to 10
weeks.
The Pfizer‑BioNTech COVID-19 Vaccine does not contain preservative. The vial stoppers are
not made with natural rubber latex. For the 10-μg RNA dose, each 1.3-mL filled via vial must be
diluted with 1.3mL 0.9% sodium chloride for injection to provide 10 doses at 10 μg RNA / 0.2 mL
Injection volume. After dilution, the vials should be stored at 2°C to 25°C and should be used
within 12 hours.
6 EUA REQUIREMENTS, GUIDANCE AND CONSIDERATIONS PERTAINING TO COVID19
VACCINES
6.1 U.S. requirements to support issuance of an EUA for a biological product
Based on the declaration by the Secretary of the U.S. Department of Health and Human
Services (HHS) that the COVID-19 pandemic constitutes a public health emergency with a
significant potential to affect national security or the health and security of United States citizens
Looking forward to it!