My testimonies and trip to the New Hampshire Capitol
I hope bits of the testimonies, or the official graphics from CDC and FDA might be helpful in other states, or in conversations
I am just back from a 520 mile round-trip I made to Concord, NH in 29 hours. Very tiring. Too many cars behaving badly. I had been asked by a nonprofit to provide testimony on a vaccine bill, and eventually 1 testimony became 4 testimonies.
But I wanted to talk about paying tolls. I don’t use an EasyPass (some states call it something different) because I decided the government did not need to be following me whenever I drive on toll roads.
Now, I did read once that the USG makes the car manufacturers put a GPS device in every car so they can follow you that way, but I had hoped my 2011 model might be old enough that it perhaps evaded the government-ordered GPS. (Admittedly, I do use a GPS when I don’t know where I am going—but now I will turn it off the rest of the time.)
I drove at night in both directions. What I noticed on this trip, but had not noticed on other trips, was that my car was being photographed as I went though the toll booths—because there were no other cars around, and quick flashes of light occurred right after I had paid. I think it was a photograph. What else could it be? I think the USG does not want people who still pay tolls in cash to evade the surveillance by EasyPass.
The GPS device, if in fact it has been installed on all newer model cars (even those without the dashboard GPS) would be a redundant way of keeping track of us. I think they like redundancy.
Anyway, below is my first testimony on Feb. 16.
Testimony of Meryl Nass, M.D. before the Health, Human Services and Elderly Affairs Committee.
Honorable Chairpersons, Members and Senators,
My name is Dr. Meryl Nass. I am testifying in favor of HB575: Should New Hampshire promote, purchase or distribute vaccines that have not undergone human trials?
I am a Maine physician and expert on anthrax, bioterrorism and vaccine safety. I have given 6 Congressional testimonies on these subjects. I was the first person in the world to show that an epidemic was the result of biological warfare.
A vaccine that has not completed human trials is an unlicensed, experimental vaccine--with one exception, seasonal flu vaccines.[1] I would ask that you amend the language of this bill so that influenza vaccines are the single listed exception.
First, it is important to know that neither safety nor effectiveness of vaccines (as well as drugs) can be determined from animal studies. Animal responses are variable, and do not predict human responses. This is why the FDA requires that 3 successive clinical trials in humans must be performed, after animal studies are completed, before a vaccine or drug can be licensed.
According to authors at GlaxoSmithKline:[2]
Before a vaccine is administered to humans, vaccine manufacturers undertake extensive safety evaluation of individual vaccine components and of the final formulation to be administered. Raw materials must be of the highest possible purity and quality... [5]. The vaccine components and the final product are tested in the laboratory for purity, sterility, potency, consistency, activity and stability (described in more detail by [46]). Many of these tests are conducted in the laboratory, and many, such as tests for efficacy, toxicity, safety and effects on reproductive health, are conducted in animal models.
After licensure, all vaccine lots must pass a rigorous array of quality control tests that are agreed on by regulatory agencies (both the authority responsible for the jurisdiction where the manufacturer is based, and the authority [or authorised delegate] on the receiving country), before they can be released. During manufacturing an individual vaccine will undergo multiple non-clinical, toxicology and safety tests (sometimes numbering in the hundreds) before being released for use in humans. New production sites need to be inspected and approved before starting their activities, after which they are regularly inspected and audited by regulatory agencies. Production sites can undergo many inspections in a year, since each individual country’s authorities may decide to inspect the facilities at any time.
The safety of an individual vaccine is continuously monitored in humans throughout its clinical development, culminating in large Phase III studies which may be designed to assess specific safety outcomes (Garçon et al. in this issue). Although smaller pre-licensure safety packages may be submitted in settings of public emergency, such as pandemic influenza, or with new vaccine formulations of existing vaccines, most new vaccines are submitted for license approval with an extensive safety data package containing detailed safety information from pre-clinical studies and from the thousands or even tens of thousands of individuals that received the vaccine in clinical trials....
According to the American College of Physicians,[3]
"Vaccine development is a long, complex process, often lasting 10-15 years and involving a combination of public and private involvement...Vaccines are developed, tested, and regulated in a very similar manner to other drugs. In general, vaccines are even more thoroughly tested than non-vaccine drugs..."
"Vaccine licensing is a lengthy process that can take 10 years or longer. The FDA requires that vaccines undergo three phases of clinical trials with human subjects before they can be licensed for use in the general public."
"Only after the FDA is satisfied that the vaccine is safe is it licensed for use in the general population.
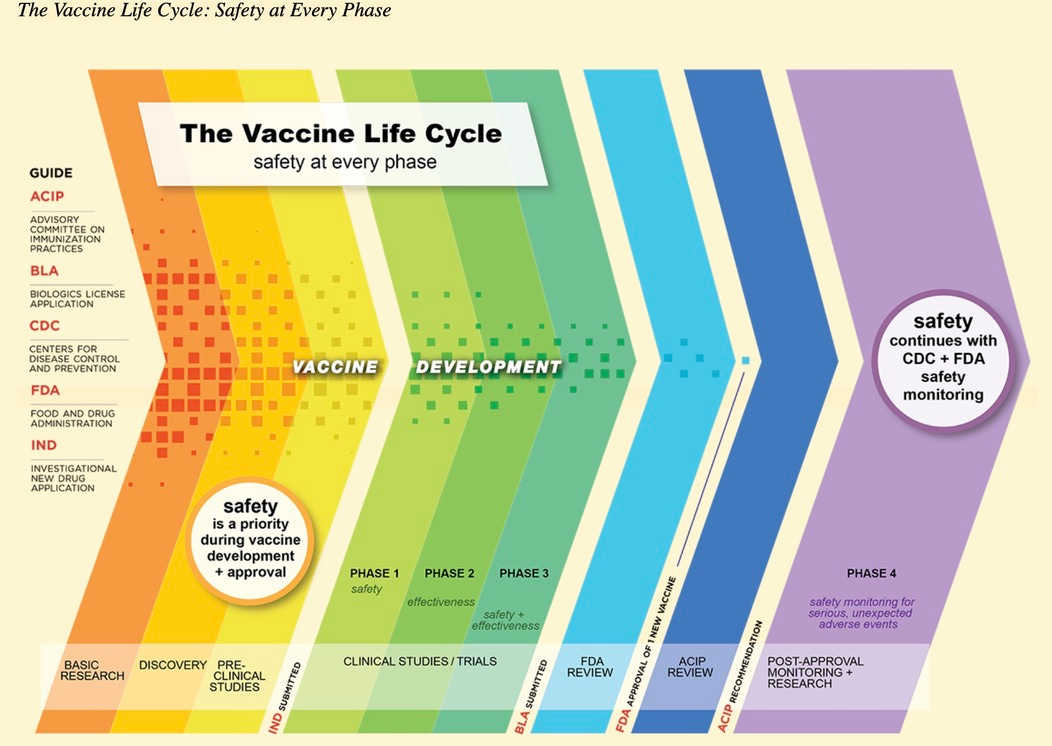
I have provided you a CDC graphic (footnote 4) to show the many stages that must be completed to get a vaccine approved. When regulators tried to skip these stages, usually serious trouble arose.
Why would any state government provide its citizens with a drug or vaccine that had not been fully tested and found to be safe and effective? The only reason would be a serious medical emergency where no licensed therapy is available.
Let's see how that has worked out each time it has been tried.
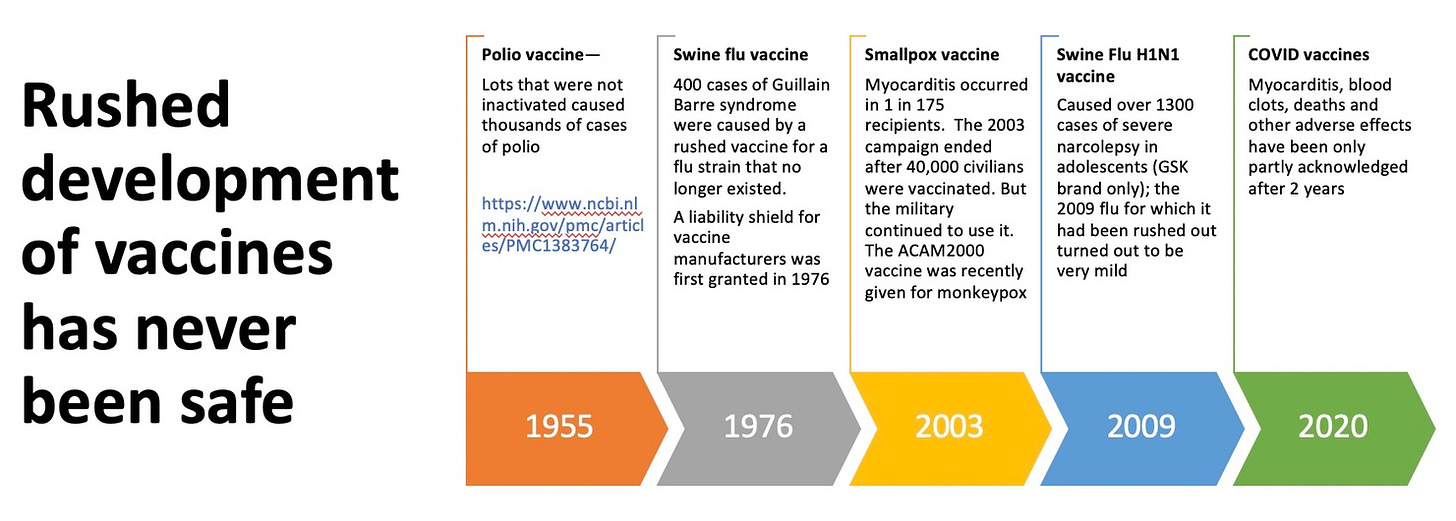
I've provided a graphic with 5 historic examples of the unanticipated problems that arose when inadequately trialed vaccines were rolled out for general use.
In the 1955 'Cutter incident,' 40,000 children got polio from an improperly inactivated vaccine.[5] In 1976, 400 Americans got paralytic Guillain Bare syndrome, 25 died and the swine flu program ended.
Since 2020, thousands of Americans have developed myocarditis from inadequately tested and unlicensed COVID vaccines that never did complete human testing.
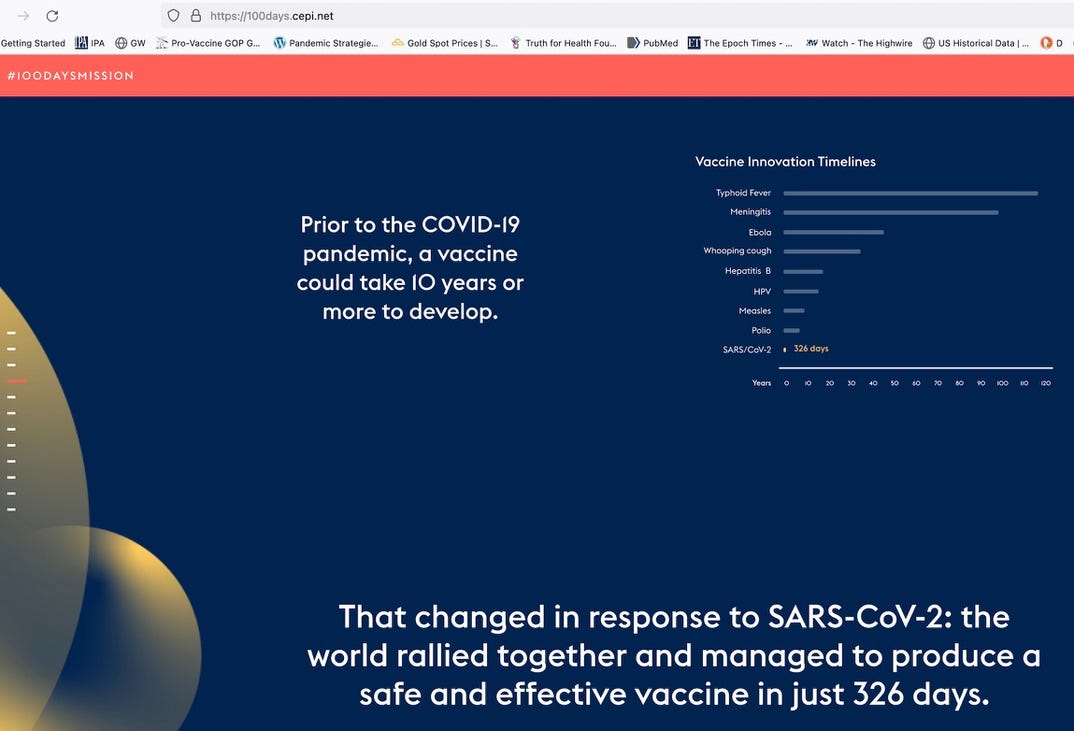
The Coalition for Epidemic Preparedness Innovations, also called CEPI, funded by the Gates Foundation, Wellcome Trust, and 32 countries including the US,[6] promises to roll out vaccines for the next pandemic in only 100 days. I can assure you that there will be no time for human testing or licensure.
CEPI compares the time it took to develop prior vaccines--usually decades--to their plan. With funding by so many countries, it is a near certainty that hurriedly rolled out, untested vaccines will be used. Realize what has already happened in the vaccine space, and what may be coming next.
If you don't want New Hampshire's citizens to be among the guinea pigs for those vaccines, please vote for this bill.
New Hampshire can protect its citizens from precipitous and reckless medical experiments by simply requiring that all vaccines except seasonal flu vaccines go through human clinical trials before being rolled out for mass use. Which is only what the law requires to give vaccines a license and call them "safe and effective." Citizens of the Granite State deserve no less.
The times are perilous. We, the people and the professionals, need your protection. Please pass HB575.
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4947948/ An overview of the regulation of influenza vaccines in the United States
[2] https://www.sciencedirect.com/science/article/pii/S0264410X16309744/ Vaccine safety evaluation: Practical aspects in assessing benefits and risks
[3] https://historyofvaccines.org/vaccines-101/how-are-vaccines-made/vaccine-development-testing-and-regulation
[4] https://www.cdc.gov/vaccinesafety/ensuringsafety/history/index.html
[5] https://www.washingtonpost.com/history/2020/04/14/cutter-polio-vaccine-paralyzed-children-coronavirus/
[6] https://cepi.net/get_involved/support-cepi/





I'm sure your state is sorry they ever pulled your licensure, and unleashed an archangel of truth!
You a badass Ms. Meryl. We love you!