Regulation using mock-ups: how Finland's 40,000 doses and the EU's 665,000 doses of bird flu vaccine were approved with no human data--UPDATED July 16.
With an option for 40 million doses. This is a long post because I was asked for more details yesterday by a doctor in Finland
Once upon a time a scheme was concocted for a future dire emergency, especially one involving a future bird flu (influenza A) virus. Bird flu, of course, could become an existential threat. Only the scientists could save us. And only the lawyers could figure out how to get around the existing laws, to allow the scientists to roll out vaccines without testing or going through normal regulatory processes.
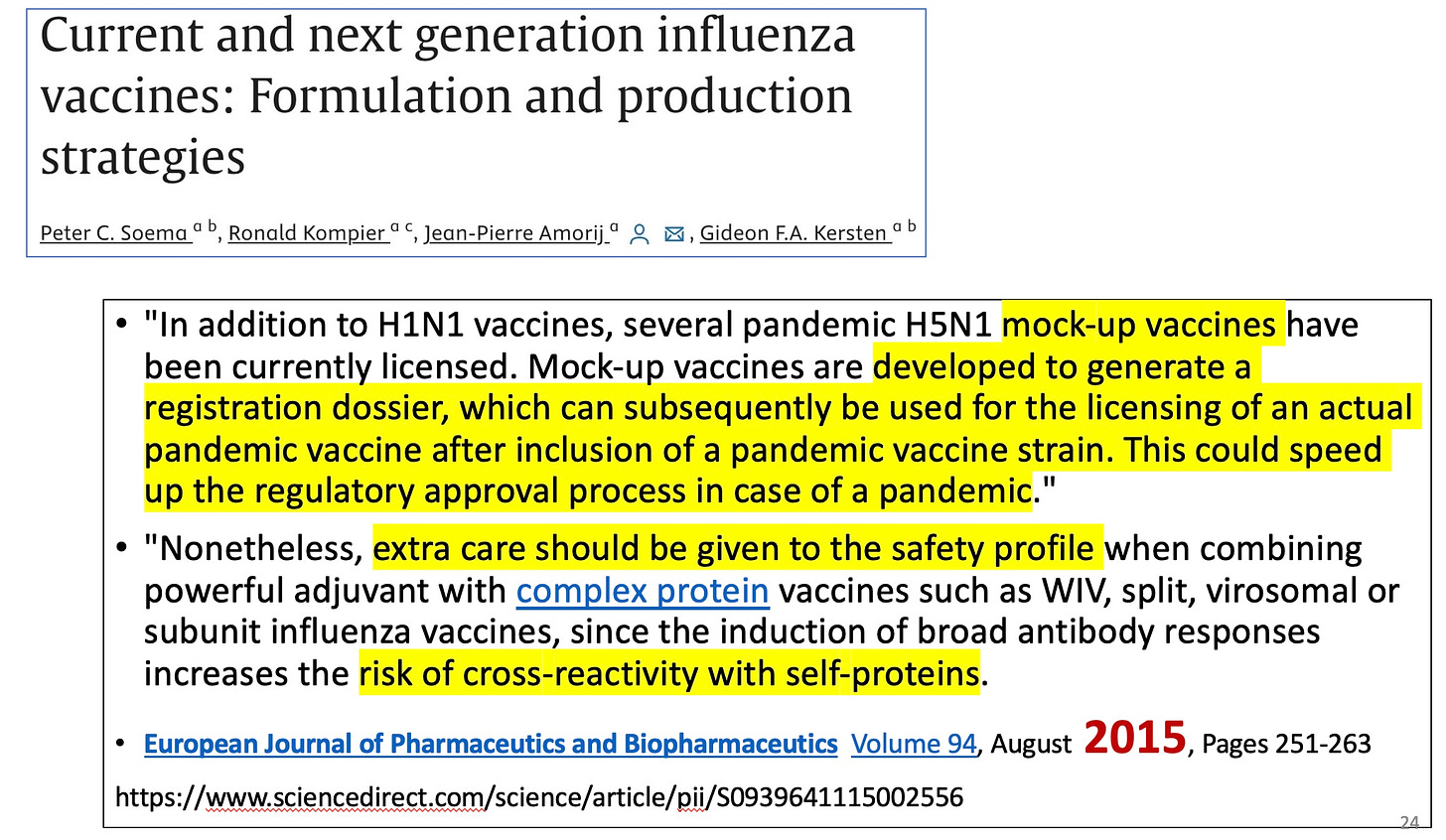
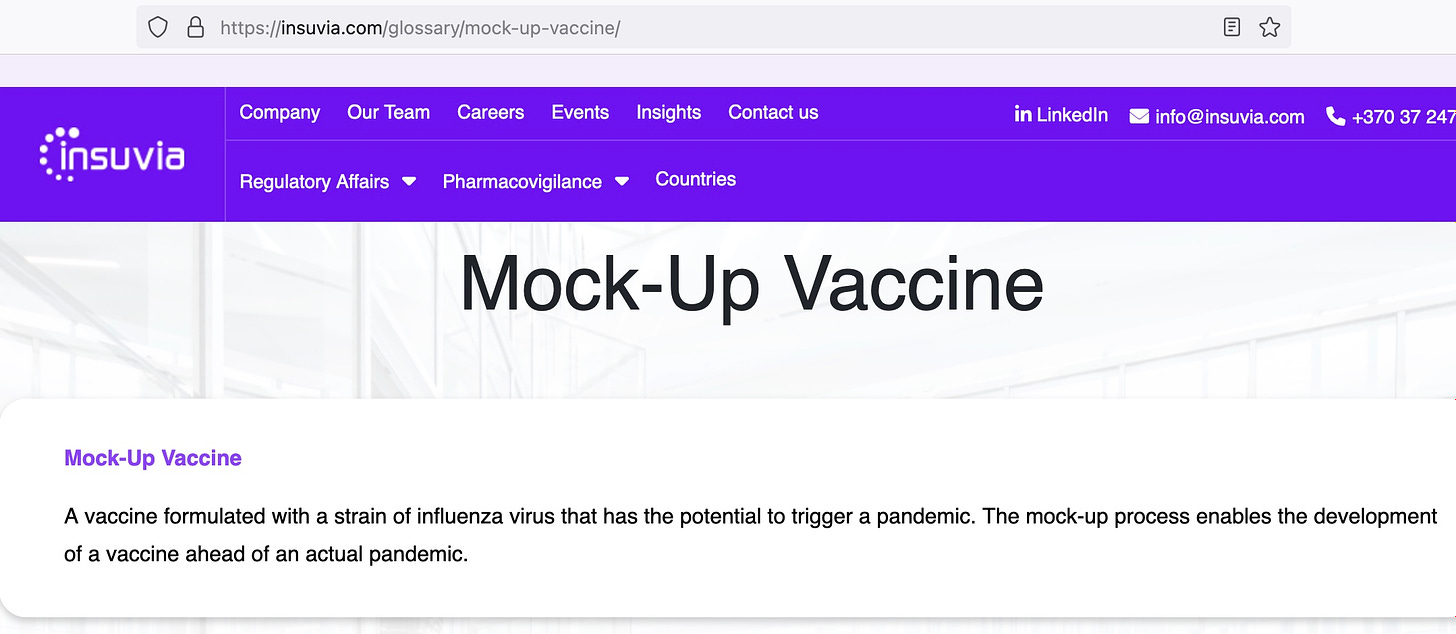
Thus the mock-up vaccine was born, where a prototype vaccine is made, but is not intended for use. It goes through the motions of a regulatory process, but no one really cares about it since it is not intended for people. At some future time, a similar vaccine, only different from the mock-up or prototype or prepandemic vaccine (these imaginary vaccines have many names to keep us confused) because it uses 1 or more different antigens, is developed quickly to respond to a pandemic. Then the regulators slap a license or an EUA or authorization on the new vaccine, based on the approval of the mock-up vaccine. Even if the mock-up vaccine may have been deadly.
If this sounds crazy, that’s because it is. But it is true:
By 2005 Congress was told that we needed to be able to fast-track pandemic vaccines and give them liability protection as well.
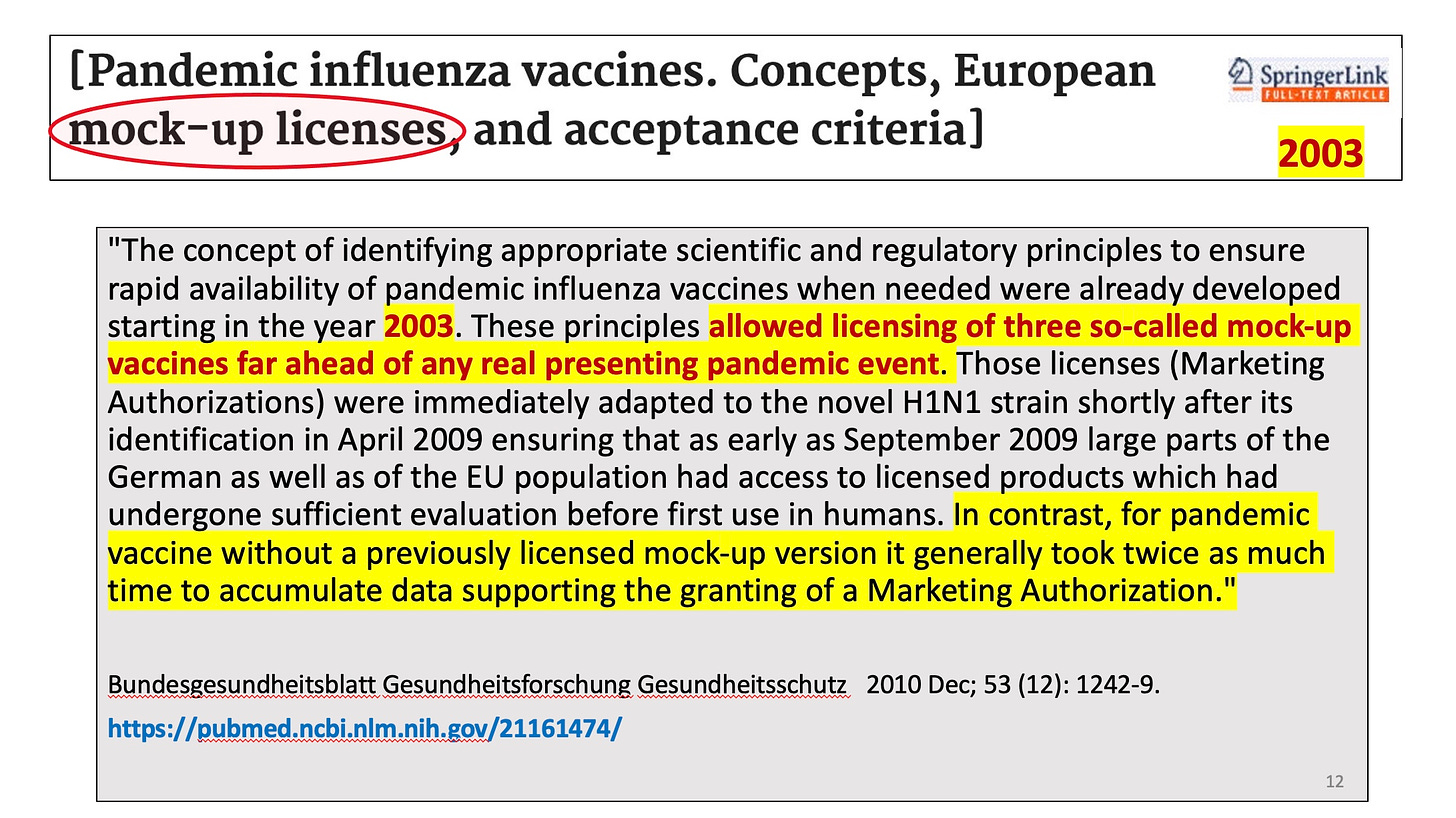
By 2007 both the US and the EU approved initial mock-up H5N1 bird flu vaccines:
There was absolutely no scientific sense, no logic, no regulatory standard that made this okay.
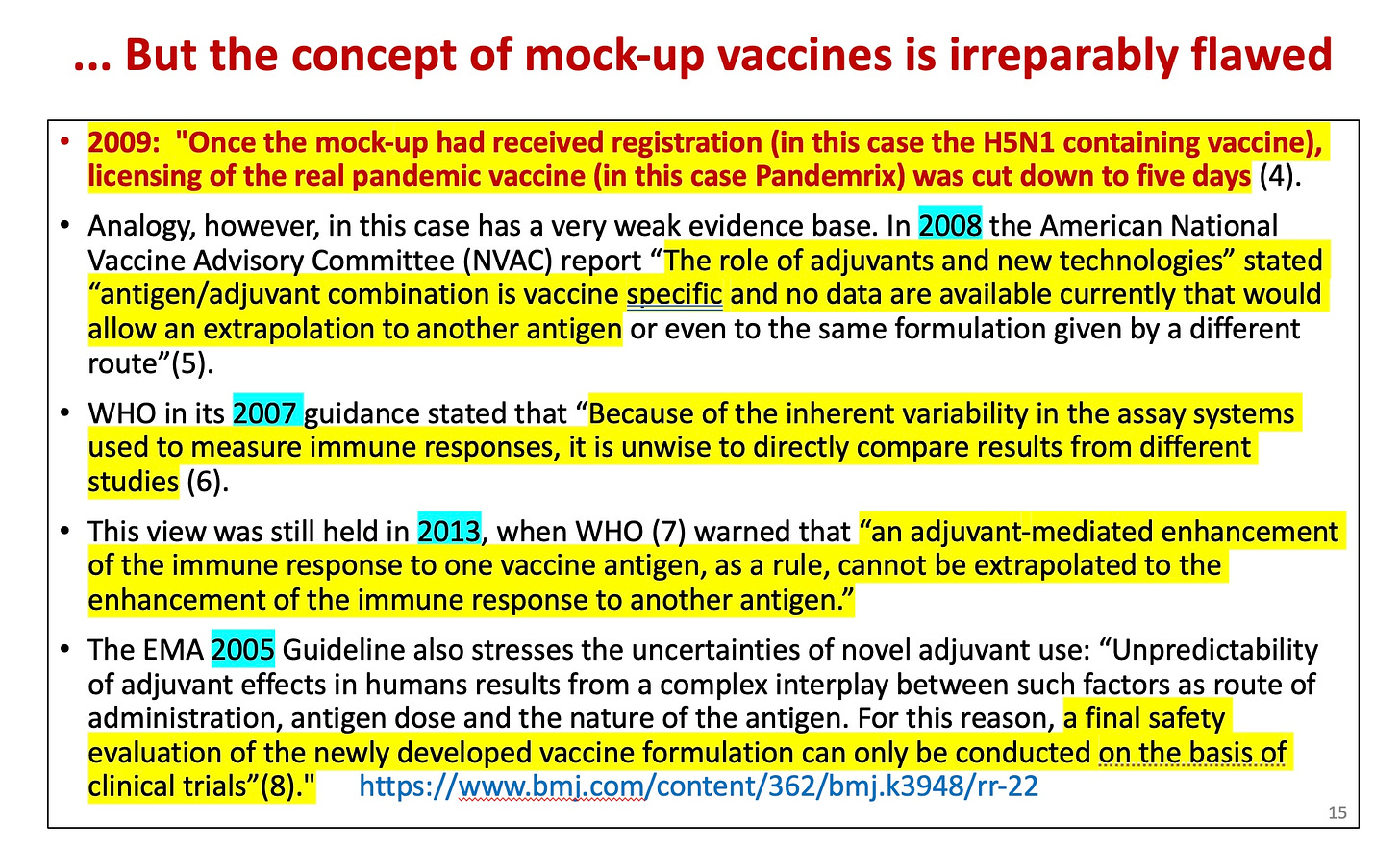
Dr. Tom Jefferson, who headed the Cochrane vaccine study group for may years, pointed out that the mock-up (recently the process underwent a name change to “pre-pandemic”) vaccine approach was entirely unjustified in the following communication to the BMJ.
He cited the WHO, the EMA (Europe’s FDA) and a US federal advisory committee as references.
[In fact, using a mock-up vaccine had allowed the GSK Pandemrix vaccine (associated with over 1300 cases of severe narcolepsy in Europe and more than twice as many other serious side effects as alternative vaccines for the 2009 swine flu) to be licensed after a 5 day review!]
None of this mattered. A legal maneuver had been created to get untested vaccines into millions or billions of arms, and there was no going back!
_______
Below you can see how this procedure was used for the 665,000 doses of Sequirus H5N8 bird flu vaccine purchased by the European Union. The original mock-up was named Aflunov, licensed in 2010.
An earlier version of Sequirus’ 2024 bird flu vaccine was okayed by the EMA in 2023 as a spin-off from the original Aflunov license. Months later, the antigen component was updated to use the FDA’s H5N8 Astrakan-like strain antigen, and this second spin-off from the Aflunov license was approved in April 2024.
Both the 2023 and 2024 authorizations of different Sequirus vaccines were based on the original Aflunov mock-up vaccine. Here is how the 2024 differs from the 2023 version. https://www.ema.europa.eu/en/documents/procedural-steps-after/zoonotic-influenza-vaccine-seqirus-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf
Here you have the legal explanation of how the 2023 vaccine was shoehorned into the much older Aflunov vaccine approval of a different vaccine: https://www.ema.europa.eu/en/documents/assessment-report/zoonotic-influenza-vaccine-seqirus-epar-public-assessment-report_en.pdf
MAH stands for the marketing authorization holder, which is CSL Sequirus, the manufacturer.
1. Background information on the procedure
1.1. Submission of the dossier
The applicant applied for the following indication: Active immunisation against H5 subtype of Influenza A virus.
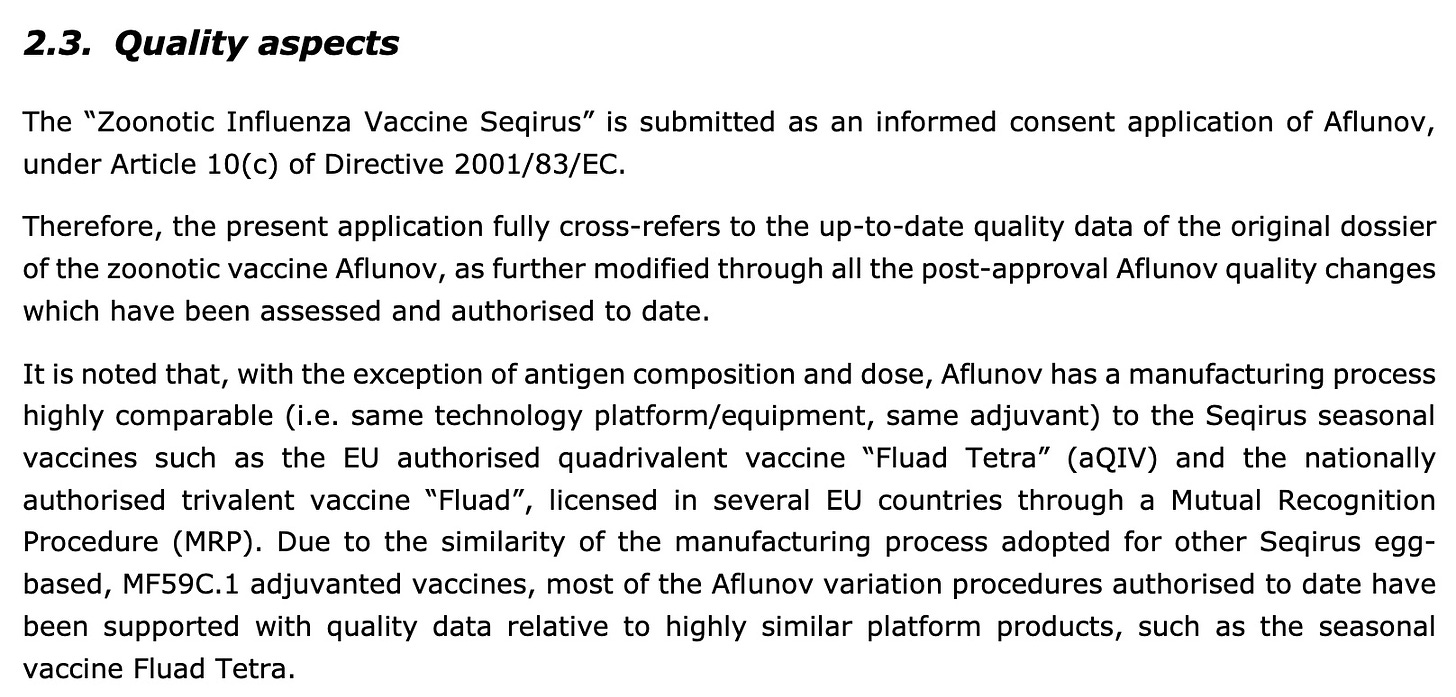
The present application pertains to the informed consent procedure according to Article 82(1) of Regulation (EC) No 726/2004 aiming to duplicate the existing marketing authorisation for the H5N1 zoonotic vaccine Aflunov (MAH: Seqirus S.r.l.), into a new vaccine named “Zoonotic Influenza Vaccine Seqirus” (Applicant: Seqirus S.r.l.).
1.2. Legal basis, dossier content
The legal basis for this application refers to:
Article 10(c) of Directive 2001/83/EC – relating to informed consent from a marketing authorisation holder for an authorised medicinal product.
The application submitted is composed of administrative information, quality, non-clinical and clinical data with a letter from a MAH (Seqirus S.r.l) allowing the cross reference to relevant quality, non-clinical and/or clinical data…
2. Scientific discussion
2.1. Problem statement
The present application pertains to the informed consent procedure according to Article 82(1) of Regulation (EC) No 726/2004 aiming to duplicate the existing marketing authorisation for the H5N1 zoonotic vaccine Aflunov (MAH: Seqirus S.r.l.), into a new vaccine named “Zoonotic Influenza Vaccine Seqirus” (Applicant: Seqirus S.r.l.).
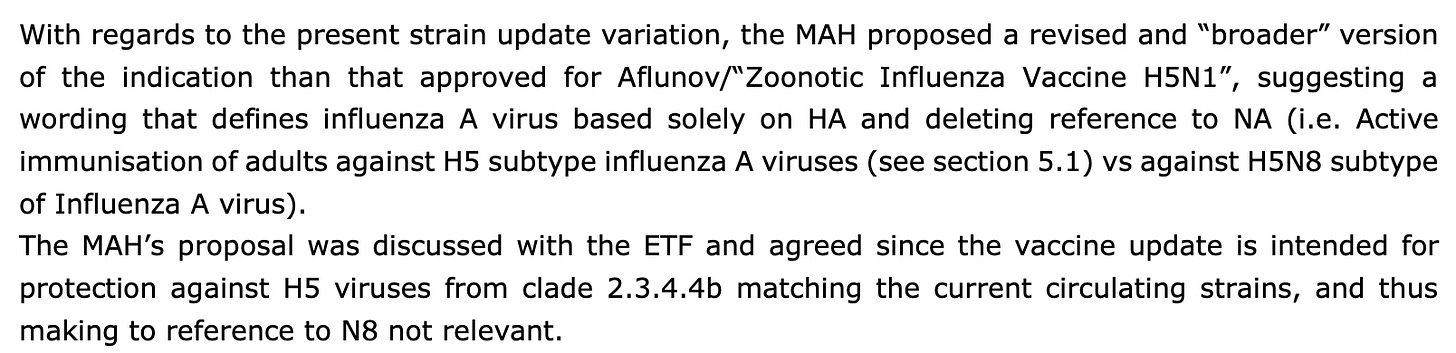
The reason behind the present informed consent application procedure is that Seqirus intends to continue the marketing of the currently authorised Aflunov H5N1 vaccine and, in parallel, to introduce a new H5N8 vaccine targeting a new clade (2.3.4.4b) which has become dominant in various world regions. The risk for occupationally or otherwise exposed groups to avian influenza-infected birds or mammals is assessed as low to moderate. The candidate virus subtype which has the greatest coverage against the avian viruses of concern which are currently of clade 2.3.4.4b is A/Astrakhan/3212/2020 (H5N8) clade 2.3.4.4b.
Therefore, following duplicate license granting, Seqirus intends to introduce a strain modification for the Zoonotic Influenza Vaccine Seqirus in order to target the new clade, while maintaining the same strain for the current Aflunov license…
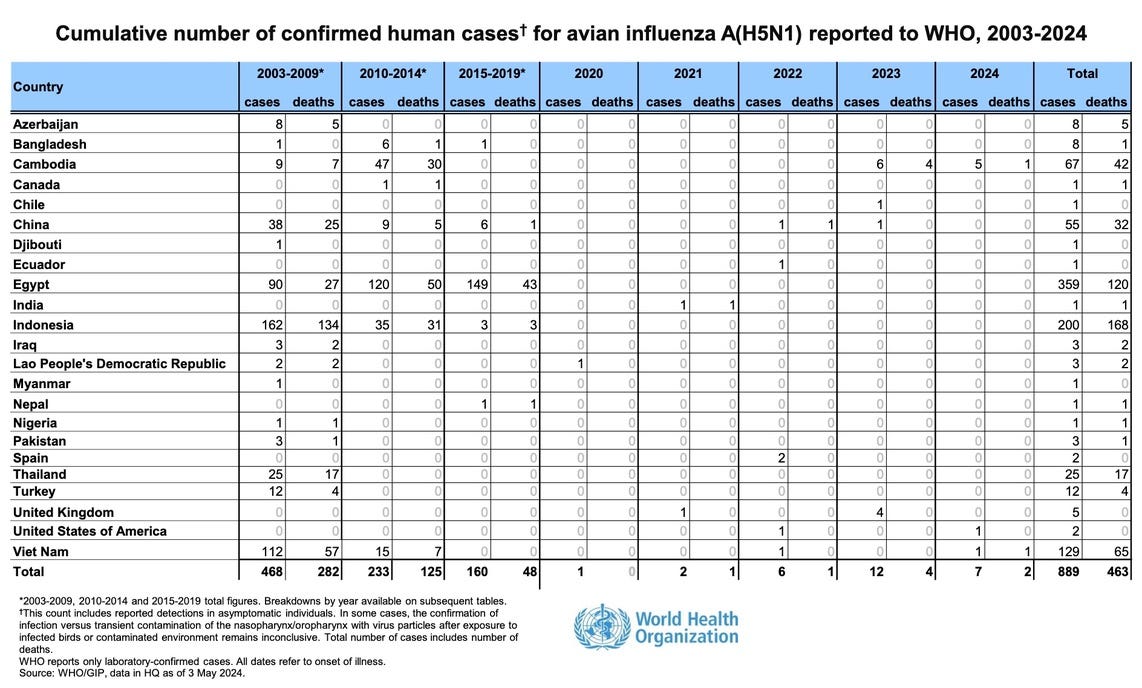
The reported mortality rate of highly pathogenic H5N1 avian influenza in a human is high and according to WHO reaches 60% of cases. However, there is some uncertainty as people with milder symptoms may not seek treatment.
That is the justification: we are dealing with a deadly disease, so we have to cut corners. Except bird flu has mutated to a barely noticeable form. And is no longer deadly. WHO claims there have been only 8 human deaths due to H5N1 bird flu since 2020, worldwide.
Here’s a bit more from the 2023 license justification. I must emphasize that the MF59 squalene-containing adjuvant in this vaccine has well known toxicity.
Here is the detailed, 45 page report of the EMA on the vaccine the EU has now purchased for 16 countries:
“No clinical data” means the vaccine was not going to be tested in humans before licensure. As you can see, the regulators themselves bolded this nugget.
The paragraph below caught my eye, because the term multibasic when used for SARS-CoV-2 referred to the spike cleavage site. The regulators are concerned that a similar multibasic cleavage site might have been inadvertently left in the vaccine. The vaccine antigen was developed at FDA’s Center for Biologics (CBER). The regulators wanted a lot more information from FDA. They detail asking for it but not receiving it.
Several paragraphs down, the regulators confirmed my suspicion. Do some authors use the term “multibasic trait” (as I believe the Proximal Origin authors did) to confuse readers unfamiliar with the term? But whoever wrote the following paragraph, on page 7, was more explicit:
Here, the regulators decided that because the neuraminidase of the H5N8 was not a match to the H5N1 strain circulating, they should simply omit mention of the “N”:
The study below looks like the first human data for this vaccine, to be obtained from people who are receiving the vaccine in Finland right now. I am troubled by the use of the term “reactogenicity” as it usually refers to only acute, minor reactions rather than serious, later-onset adverse events. This means the regulators will be interested in reports of immediate (but temporary) reactions to the vaccine, but apparently uninterested in later-onset, more serious and potentially permanent adverse events. “Passive” surveillance means they will not go looking for reactions, which is termed active surveillance, but will accept reports generated spontaneously.
That is not how you collect data on a totally new vaccine that was never given to humans before. But it IS what you do when you are trying to avoid learning any bad news about the vaccine. It is not an acceptable procedure.
The EMA also wants to see US data, after the European vaccine has been approved, but the US data will be derived from a cell-based production process, while the European vaccine is being produced using eggs, so they are not considered identical vaccines. There is no telling when US data may be available.
The US trials are here and here, and the clinicaltrials.gov websites do not present any US results, though the first trial was completed a year ago.
The best that Sequirus could do for safety was inject 20 ferrets, who were examined at 1, 2 and 3 days post injection and seemed fine at those time points. The ferrets developed antibodies, so an assumption was made that the vaccine was immunogenic, i.e., good enough. Ferret data cannot provide safety or efficacy information and should only be a precursor to human studies, from which detailed data are collected.
The Finns appear to want to have their cake and eat it too, as the Americans did with the COVID vaccines: give people a licensed drug that they are misinformed has gone through proper regulatory procedures, then try to collect some limited data from the recipients, but much less than you would collect in an actual clinical trial. That way you appear to be giving medical care, and you also appear to be conducting a trial, but neither is done properly.
Finally, the regulators say they have recommended that Sequirus take additional measures—but have redacted what those recommendations are. Why would they do that unless they don’t expect Sequirus to comply with the recommendations. Are they requirements or recommendations?
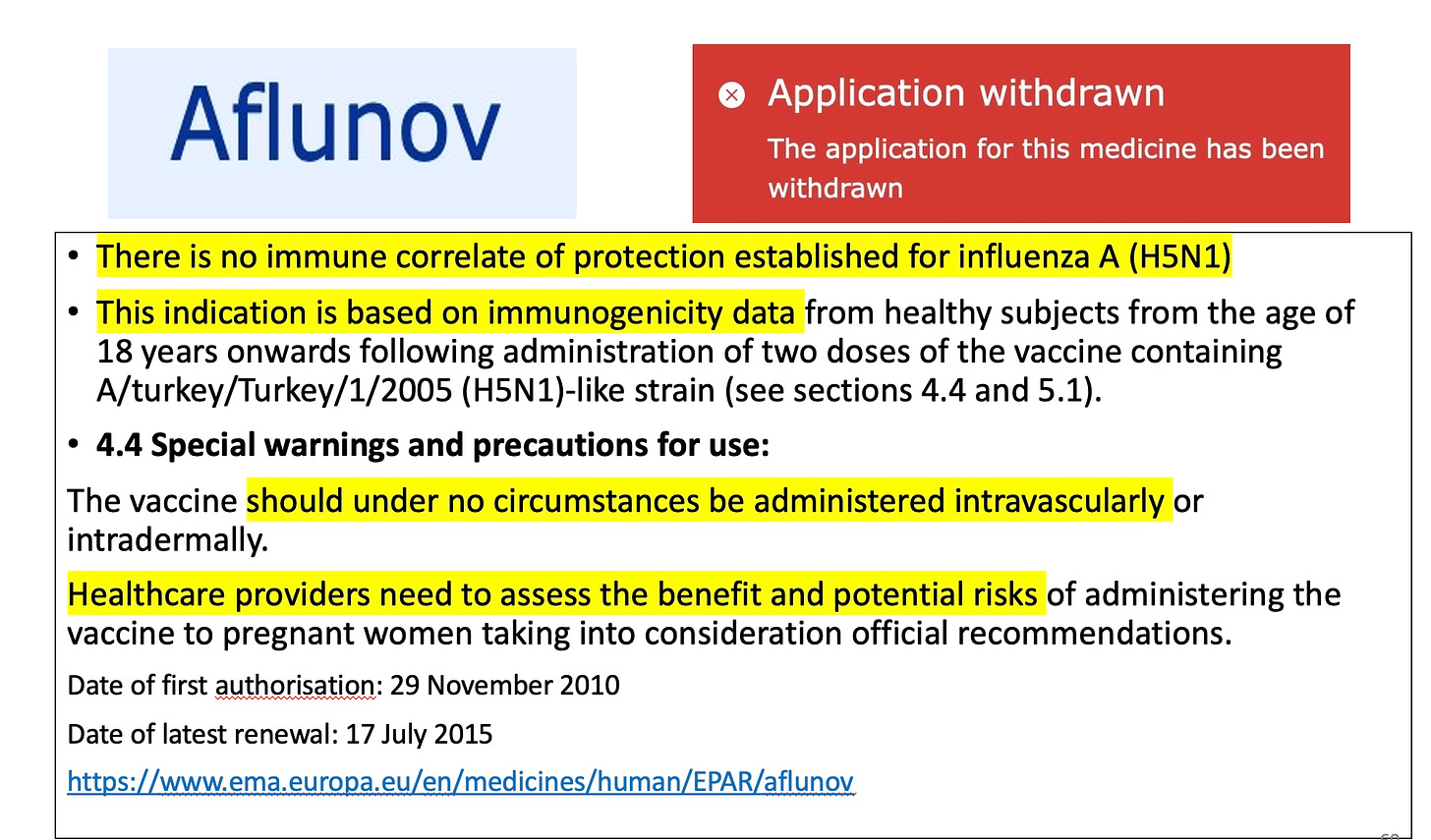
So there you have it. Aflunov was approved in 2010 according to this document, essentially not used, and yet its original approval is the basis for a new vaccine to be licensed without testing in a single human being. Welcome to 21st century regulation.
And at one point Aflunov lost its license. But let’s not mention it.
Here is a list of Finnish people recommended to get the vaccine. The usage of the term “Precautionary Principle” is the opposite of its real meaning, as it means to avoid products until they are clearly beneficial and the risks are known.
Below, the vaccination program is justified by the curious One Health concept. The vaccination program will enhance planetary health, even if it might provide more harm than benefit to vaccine recipients. Morality in the New World Order.
UPDATE July 17: Here is a video made by Sami Antinniemi, most in Finnish, about bird flu and the bird flu vaccine, which includes his interview with Hanna Nohynek, Finland’s chief vaccine officer, and an interview with me.
https://rumble.com/v570od1-kantoraportti-lintuinfluenssarokotteen-turvallisuutta-metsstmss.html














Insanity
So….money laundering by greedy killer clowns.
Lather
Rinse
Wash
Repeat