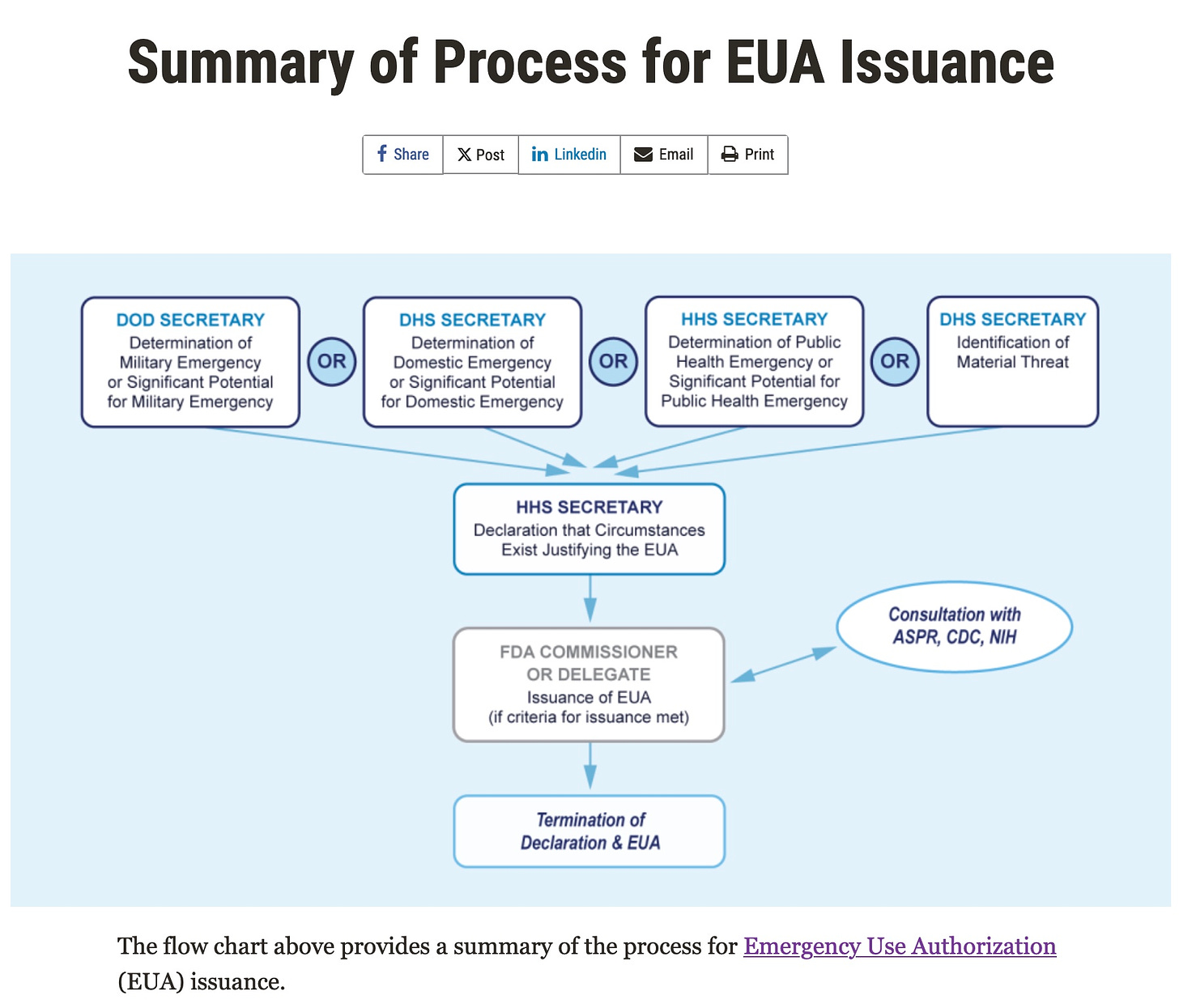
The process for issuing EUAs is (deliberately) complicated; only the 1st step of 3 was taken by Sec. Becerra
I will explain the 3 steps and correct the FDA
This article is mostly based on this FDA document: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-medical-products-and-related-authorities#scope
Here is the actual declaration of Sec. Becerra.
Here is the Federal Register explanation of the 3 steps and how this declaration expands to cover all bird flu viruses.
Step 1: The Secretary of DOD, HS or HHS declares an emergency or the potential for an emergency due to a particular agent *
Step 2: The HHS Secretary, in consultation with his Assistant Secretary for Preparedness and Response (ASPR), the NIH Director and the CDC Director if feasible, issues a declaration/ authorization that a product may be effective at treating, preventing or ameliorating a disease or condition caused for the agent in Step 1
Step 3: The FDA Commissioner issues an EUA for the product, invoking a broad PREP Act liability shield:
The mistake in the graphic below shows the consultation between the FDA Commissioner and the ASPR, CDC and NIH. The arrow should instead show that consultation between the DHHS Secretary and the ASPR, CDC and NIH, which precedes the 2nd step declaration.
The FDA explained this process in a guidance document for industry published in the Federal Register in 2017:
Section 564 of the FD&C Act, as amended by PAHPRA, permits the Commissioner to authorize the emergency use of an unapproved medical product or an unapproved use of an approved medical product for certain emergency circumstances after the Department of Health and Human Services (HHS) Secretary has made a declaration of an emergency or threat justifying emergency use. That declaration by the HHS Secretary must in turn be based on a determination of an emergency or potential emergency or material threat associated with the CBRN agent by, respectively, the Secretary of Homeland Security, the Secretary of Defense, or the HHS Secretary.
It is this (first) declaration that HHS Secretary Becerra made on July 18, 2024.—Nass
The [FDA] Commissioner may issue an EUA to allow an MCM [medical countermeasure] to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by a CBRN agent, or by a product used to diagnose, treat, or prevent such diseases or conditions, when available data meet specified criteria to support such uses and there are no adequate, approved, and available alternatives.
Section 564(b)(1)(C). Prior to the PAHPRA amendments, the Secretary would have made the determination that there is a public health emergency under section 319 of the PHS Act. Under amended section 564(b)(1)(C), the Secretary can make the emergency or threat of emergency determination that includes any and all of the elements required by statute (e.g., that the emergency affects national security, U.S. citizens living abroad, etc.) when making the declaration justifying the EUA under section 564(b)(1)(C). If there is an applicable section 319 public health emergency determination in place, the Secretary may conclude that any additional elements required by the statute (e.g., that the emergency affects national security, citizens living abroad, etc.) are met when issuing a declaration under section 564(b)(1).
Here is how this process is explained by the ASPR:







With an EUA declaration from HHS for whatever bird flu strains they've chosen, the FDA is now free to issue EUAs for any product whose benefits they believe exceed the risks, based on "available information."
If there's anything like IVM or HCQ that could work for bird flu, they'll have to suppress it, because there cannot be any other "adequate, approved and available" candidate product for doing what the EUA product is supposed to do.
Meryl, I don't know how you can read, absorb,and translate our Byzantine bureaucracy 's pronouncement and regulations into plain english..but I'm very very grateful you do.