Asked by CHD's Defender to comment on the FDA's failed attempt to bury a million pages on Comirnaty, I fleshed out an answer for those less familiar with the details
I want to be sure all my readers understand what is at stake in this case
IMHO FDA attempted a fraud on the court by:
Failing to produce documents it knew it was required to make public, based on the original court ruling and FDA law.
And then FDA tried to trick the court into acquiescing with its partial production as if it was the whole enchilada.
And then it came up with a lame excuse for why it had gone down this route, probably expecting the court to give it deference, as it had done during the 40 years of the Chevron deference doctrine.
As Judge Pittman noted, the language in the original ruling was crystal clear. And I would point out that the law is also clear regarding the requirement to put the entire package of documents used in a licensing decision in the public domain, once a product is licensed—yet FDA decided to attempt a fraud, regardless. Thus it must have had something big to hide.
The 75 years ploy told us FDA was very worried, and this attempt at a fraud on the court is another piece of evidence the FDA is absolutely desperate to hide its records. Which leads one to consider, will the FDA fully release all its records in response to this second court order?
What is FDA trying to hide?
FDA was desperate to issue an August license in order to impose mandates
We know from a FOIA’d set of emails that FDA’s head of the Office of Vaccines Research and Review and her deputy (Marion Gruber and Philip Krause, both long-term FDA officials with PhDs who were the vaccine officials just under Peter Marks, the head of CBER) either quit or were (more likely) fired because of their refusal to issue a license for Comirnaty before the date demanded by FDA’s acting Commissioner, Janet Woodcock, MD in August 2021.
The license was was then issued by Peter Marks—a month faster than Gruber said was possible, according to a detailed email she wrote to memorialize what sounded like an acrimonious meeting with the acting FDA Commissioner about this subject in July 2021.
The license issuance on August 23, 2021 allowed mandates to be established for college students, federal employees and the military. Without a vaccine license, FDA believed mandates could not legally be issued, according to Gruber’s notation of the meeting.
The federal government did not have the right to impose mandates on private parties, so it issued threats instead.
Once the license was issued, private parties were then urged or threatened to issue mandates as well. Hospitals, for example, were told they would lose their Medicare and Medicaid reimbursements if mandates were not issued to their employees.
Immediately after the Comirnaty license was issued (within days) mandates were issued.
Therefore, FDA probably sees itself as culpable for the issuance of mandates when it knew (according to its December 10, 2020 VRBPAC advisory meeting on the vaccine) that it did not stop transmission. By July 2021 there was strong epidemiologic evidence that the vaccine did not stop transmission. And furthermore, on August 6, 2021 CDC Director Walensky admitted to CNN’s Wolf Blitzer on TV that the vaccine “no longer stops transmission.” This admission occurred more than two weeks before mandates were issued.
Yet the ONLY scientific (and ethical) justification to impose a vaccine mandate is to stop transmission. But FDA and CDC knew the vaccine did not stop transmission when the vaccine was licensed and when it was mandated. They failed to tell the truth about this, failed to stop the mandates, and might see themselves as responsible for injuries, deaths and losses of livelihood that resulted from the mandates.
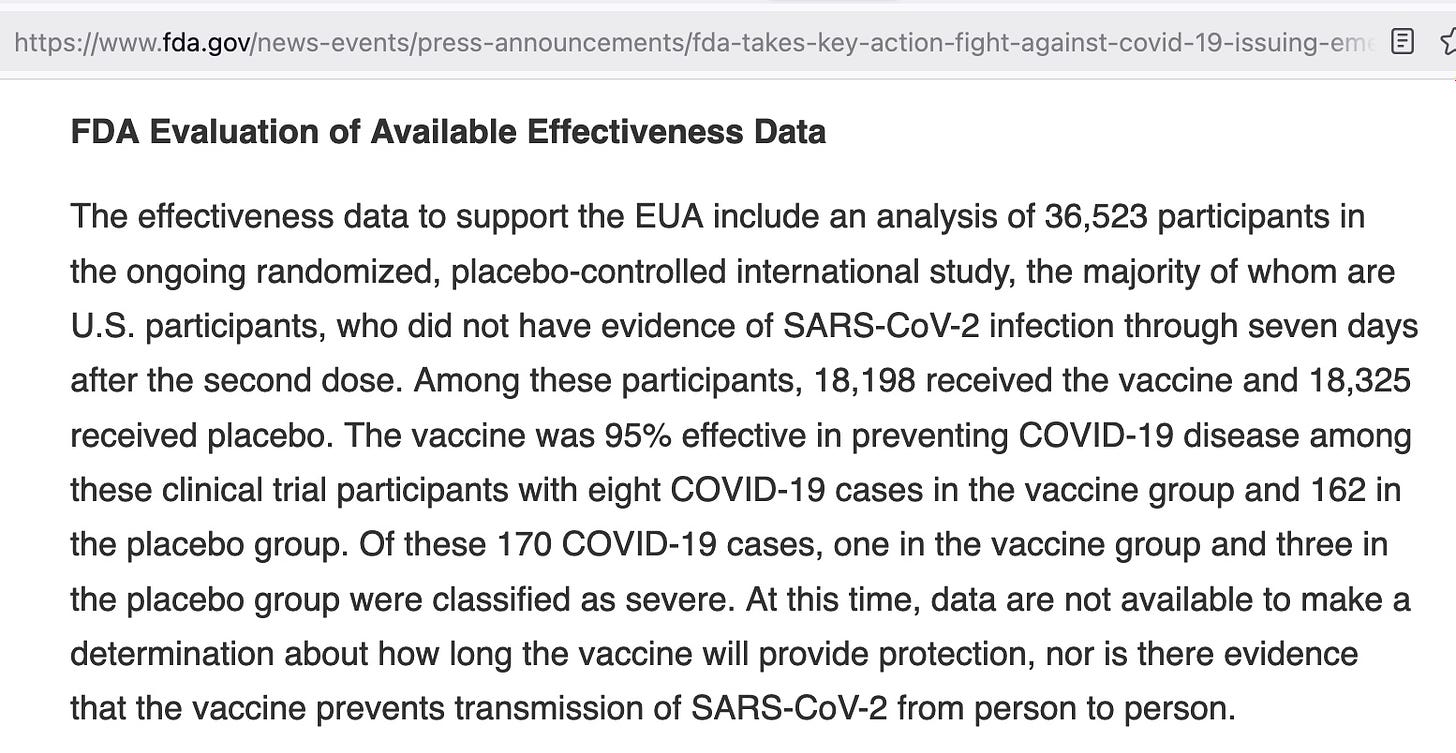
FDA also failed to require that Pfizer provide evidence the vaccine prevented transmission during initial clinical trials. Why? Below is from FDA’s Press Rlease when the EUA was issued on December 11, 2020.
FDA also learned plenty about side effects during the initial 8 months of the rollout, but failed to allow this knowledge to influence issuing the license. The FDA has still not warned the public about most of the known, serious side effects. The FDA has not balanced risk and benefit, as required.
FDA dodged dealing with the myocarditis risk by ordering BioNTech to conduct studies on myocarditis that would take several years to be completed, in its August 23, 2021 licensure approval letter. FDA claimed it was not able to study the issue itself, despite its access to many databases including VAERS, and its work with CDC (which is also charged with studying side effects). FDA has never used its ability to halt Comirnaty vaccinations for some or all age groups or demographics. And we still don’t have the results of those BioNTech studies that FDA ordered more than 3 years ago. Sixteen colleges still mandate COVID vaccinations for students.
FDA and CDC are often used as authorities by countries around the world that do not have their own drug regulatory agencies, and we know from leaked emails that FDA urged other regulatory agencies to issue their versions of EUAs before they were ready to do so. Therefore, the injured parties for which FDA might have legal culpability could extend beyond the USA.
Not only was extensive DNA plasmid contamination ignored by FDA, but also the extremely concerning presence of SV40 DNA in the plasmids—sequences that are specifically used experimentally to transport DNA into cell nuclei.
What did FDA’s internal memos say when it was brought to FDA’s attention that the vaccine was highly contaminated with DNA plasmids, and that those plasmids, with their SV40 sequences, made it very likely that the DNA would enter cell nuclei and could cause mutations? Was FDA aware of this before Kevin McKernan (working on his own dime to sequence material in the vaccine vials) informed them? It was either that FDA did not know (and was therefore negligent) or it did know and maliciously kept this information from the public.
FDA fraudulently allowed EUA product to be used, rather than licensed product, deliberately hiding this from the public. FDA used several tricks to do so. The first was a footnote in FDA’s licensure letter to BioNTech on August 23, coyly acknowledging that equivalent but “legally distinct“ vaccine (the main legal distinction being that EUAs had liability waived while licensed vaccine did not) might be used due to inadequate amounts of licensed vaccine. FDA’s second trick was to claim that certain EUA batches had been “made under BLA conditions” (which appears to be a new FDA invention) and therefore could be used instead of licensed product. After that, FDA kept quiet and EUAs continued to be used.
FDA later grandfathered in COVID vaccine boosters without any human testing in some cases, or very minimal in others, claiming it could do so because this was done with influenza vaccines. This legal justification is very questionable.
After booster vaccine versions were licensed, FDA Commissioner Califf somehow claimed that vaccine being used for its licensed indication, after the federal government had declared the end of the COVID emergency, could also have its liability waived by issuance of an EUA. This was certainly not in keeping with Congress’ intent when it passed the Project Bioshield Act and the PREP Act, is questionably legal, and there must have been plenty of memos by FDA lawyers about this particular action.
There is also the question of how the 40,000 person clinical trial was conducted and analyzed. FDA had to approve the trial protocol and Pfizer's analysis of the data. We know Pfizer fudged the study in many ways. Likely FDA analysts noted some of this bad conduct and bad analysis and were overruled by superiors.
Brooke Jackson notified FDA of serious problems at a Pfizer vaccine clinical trial site and was fired within a few hours—clearly someone at FDA told her boss at the trial site that she was a whistleblower. This is illegal. Those records should be in the Comirnaty EUA file. They will support Brooke’s Qui Tam case. FDA never investigated the trial site.
Finally, under what insane justification can COVID booster and primary vaccinations still be supported by the FDA and CDC, with all they clearly know now? Surely there will be a paper trail of disgruntled employees discussing this in the bowels of the FDA.
I suspect there is much more than this, and much I am forgetting today. I suspect the entire file must reveal who gave the orders to the FDA Commissioner and acting Commissioner to break the law, permit a charade of a clinical trial, issue an EUA and then issue a license against all logic and against FDA’s standard procedures. And one hopes it will reveal who gave the orders to FDA to cover their tracks ever since.
We will then be a step closer to learning Who Rules America.




In January, 2021, as the Pfizer shot was being rolled out, reports to VAERS were already leaping 50x higher than they had been for the previous many years. I don't use the word "spiking" because that implies that the rate would rise and swiftly fall again. No, the reports to VAERS rocketed up and stayed up for 2 years. In what universe does FDA ignore a signal like this?
Thank you Dr. Nass. As usual, you present the facts for us to absorb. You are a national treasure; actually a treasure for al of humanity.