CDC did not put the COVID vaccines on the childhood schedule today
What happens tomorrow? My liveblog with some useful additions
CDC did something strange today: they added COVID to the Vaccines for Children program, which is a federal entitlement program that pays for vaccines for kids who don’t have other insurance. But it was not necessary to do this yet, as CDC cannot buy EUA vaccines. The COVID vaccines (as well as monoclonal antibodies, some other drugs, PCR and rapid tests) are unlicensed experimental products. EUA vaccines cannot be sold. The government buys them and your taxes pay for them.
CDC spends about 5 billion dollars a year to buy vaccines on the childhood schedule for kids on medicaid or without insurance from the manufacturers. Here is the price list.
So the vote today meant that once the COVID vaccines are sold commercially, they will be included in the Vaccines for Children program. At some time that was unspecified.
There was no clear explanation why this vote was held today, when CDC is jumping the gun. Did the CDC switch out a vote on adding COVID vaccines to the childhood schedule for a different vote at the last minute?
The CDC briefer, Jeanne Santoli, who was trying to explain why the members were voting on this, gave a very short and non-explanatory talk. I just timed her and it lasted all of 3 minutes. It was totally unlike the presentations by other briefers. Jose Romero, who is the Director of CDC’s National Center for Immunizations and Respiratory Diseases, followed her and said this is not about the childhood schedule. You can listen to them and read the slides at 6 hours 31 minutes and draw your own conclusions.
The more I think about it, the more I believe that the VFC vote was a last-minute plug-in and that CDC cancelled a vote on adding the COVID vaccines to the childhood schedule, which would cause them to be required for most American kids to attend school. The midterms are coming, after all. And people are watching.
Below is my live blog.
merylnass:It is October 19, 2022 and the CDC has scheduled a 2 day meeting of its advisory committee. However, CDC has been cagey about what they plan to change in the childhood schedule.
merylnass:There is a large vaccine lineup to be discussed:chikungunya, pneumococcal vaccines, flu vaccines, meningococcal vaccines, RSV vaccines, monkeypox vaccines and dengue vaccine.
merylnass:I had to give it a couple of extra clicks to get the show to start at https://video.ibm.com/channel/VWBXKBR8af4
merylnass:Does anyone have any memory of when the CDC or ACIP responded to comments at any time in the past? CHD encouraged comments regarding the authorization of COVID vaccines for the youngest kids--I think about 40,000 comments were sent. CDC was silent. Crickets. No response.
merylnass:Dr. Nirav Shah, the head of the Maine CDC, a smart narrative pusher, has now joined the ACIP. Dr. Kevin Ault is now gone--did he criticize the vaccines for COVID?
merylnass:Katherine Paling, Cybil Cinneas, Camille Kotton, Sarah Long, Nirav Shah,Matt Daley, Oliver Brooks, Lynn Bhata RN, Jamie Lehrer, Wilbur Chen, Keipp Talbot, Beth Bell, Ms. Veronica McNally, Dr. Sanchez, Grace Lee.
merylnass:These are the voting members. Now they go through the ex officio, nonvoting members.
merylnass:The CMS and FDA members are MIA. That is odd. Office of ID and HIV_AIDS policy is also missing—he came late. Sean O'Leary is an ex officio and he also made a comment to the last meeting, strongly in favor of covid vax for kids.
No AMA rep. Normally that would be Dr. Sandra Fryhofer. She came late. Stinchfield also made a comment last time--I'd wager the CDC asked its ex officios to weigh in and give it some support last time. Society for adolescent medicine is also missing. It is odd that so many are missing, especially the FDA member who might be needed for the discussions.
merylnass:There are several pneumococcal vaccines and clarification for which should be used when is needed. Pfizer and Merck have 2 each for adults.
merylnass:The numbers in the names refer to the number of strains aka serotypes of pneumococcal bacteria included in each vaccine. In general, the more serotypes, the more cost.
merylnass:I will again note that CDC has a 100 million dollar media center but it seems to use a $20 dollar setup for the ACIP meetings, which are chock full of delays, disconnections, and now a ten minute break. We never see the faces of the speakers and there is no livestreaming.
merylnass:The break appears to be due to difficulty connecting the speaker. These glitches may serve to cover up deliberate glitches when someone is saying something the CDC does not want the public to hear.
merylnass:Pablo Sanchez has arrived and the system is back up. Sandra Fryhofer is here too.
merylnass:No one has acknowledged any conflicts of interest
merylnass:Anyone else finding the sound keeps cutting off?
merylnass:Best I can tell, in the silence, this will be a presentation of modelling and therefore another example of how CDC hates to use real data
merylnass:Rochelle, what's up with the $100 million media center? Suggest you try Zoom.
merylnass:Finally I refreshed the page and learned I have missed a bunch
merylnass:The guesstimate is that it costs a lot of money to save a single year of life--hundreds of thousands of dollars. Who pays the costs? Whose life is saved? If you save a 70 year old for 10 years, it might cost $4 million. Did the money come from another program or is it from moneyprinting that will debase the currency and cause inflation?
merylnass:And most of the benefits are speculative.
merylnass:How much is a life worth? In other places $100K/year or $127K/yr have been suggested. So using these models, the vaccine is too expensive given the benefits.
merylnass:Maine's Dr. Shah is the first questioner. They can't find slide 12. how can that be?
merylnass:Did anyone hear how long the vaccine lasts? Why do these people get so many different pneumococcal vaccines?
merylnass:No one understands this comparison, which is all modelling anyway.
merylnass:As western civilization ends, the science becomes unintelligible
merylnass:Let's move on to something else and maybe some of the members will be able to understand that one.
merylnass:Note that PCV 23 is the OLDEST vaccine. Its efficacy was called into question at least 15 years ago, and it causes significant inflammation when given. You would think that the broadest spectrum vaccine would be the most beneficial, but all these other vaccines have beencreated because PCV23 is not that good. Instead of replacing it, they just keep adding more kinds.
merylnass:Now the slide show fails again. CDC is such a clown show. The Keystone Cops. 13,000 employees. A 15 billion per year budget.
merylnass:The evidence to recommendations part of the discussion is the BS part, in which all sorts of fluffy considerations are thrown together to conclude whatever CDC wants the committee to conclude. Note that her slide was based on "claims data" so you cannot check the database and make sure what she is telling you is accurate
merylnass:Invasive pneumococcal disease numbers come from CDC, but there is no reference where we can look up the data and see how it was derived.
merylnass:Now she admits they used estimates for some of the numbers.
merylnass:When PCV13 vaccine was introduced, there was no reduction in disease, and in fact a slidght increase.
merylnass:Pfizer study admits the new serotypes in PCV20 only account for 3-4% of cases--not a great benefit, especially when Pfizer is telling you.
merylnass:Not sure that data gathered during the pandemic is that accurate, when people would do anything to stay out fo the hospital.
merylnass:The newer vaccines "are expected" to do better than the old PCV23--wait, what? The conjugate vaccines have been available for decades and are very expensive. They still don't know how well they work?
merylnass:Note: no PCV20 efficacy or effectiveness data
merylnass:Gee whiz, we are looking at antibodies 1 month post vax. Means nothing. And the data must suck, because they provide no numbers for us to evaluate. This is a travesty: believe what I say, as I won't show you the data.
merylnass:Now we look at anticipated benefits, since we don't have data to tell us the benefits. Even so, the benefits predicted are slim. They never tell us how the workgroup voted when there is questionable benefit. Then, anticipating no side effects, you might as well add this new 20 valent vaccine since of course all vaccines bring tremendous benefits and no adverse events
merylnass:Maybe the fact they were already vaccinated with older vaccines has something to do with benefit? Pfizer surveyed medical providers and they liked the vaccine, but liked it least for adults who are healthy and already had 2 different PCV vaccines.
merylnass:GIGO again. Complex assumptions that vary between models for the Pfizer and Merck vaccines, thus of limited value.
merylnass:Still quite expensive to gain years of life for people in their 70s and up--up to half a million per year to gain a bit more life--and this is based on modelling that is entirely unreliable.
merylnass:Now she fails to show how the work group voted--but I can assume they were not favorable
merylnass:The Affordable Care Act was a giveaway to vaccine mfrs. Once the vaccine is recommended by this committee, insurance is required to cover the cost 100%--no copay or deductible--by one year after the recommendation is made. This is how Obama was able to claim he was all in on prevention. Note that this allowed vaccine prices to skyrocket.
merylnass:Now they give Merck a special dispensation to weigh in!!! What did that cost Merck?
merylnass:He asks them to remember that the oldest PCV23 vaccine works, and "remains a good option." In other words, you don't have great data for the PCV20, and our old vaccine works (but maybe it works, unclear about that) so use us not Pfizer.
merylnass:One thing these members hate is complexity and this PCV20 vaccine addition will make a complicated schedule even more complicated.
merylnass:Dr. Brook says maybe vaccinating the kids will help the adults (and then you won't need to vaccinate the adults, since that appears to be what happened when the Prevnar vaccines rolled out.
merylnass:Dr. Long: this is difficult stuff. The work group rarely settled on a single answer, and we were a problematic group. What they should have concluded is that there is not enough benefit to go with the new vaccine, and can revisit the issue after the vaccine is used in the population, probably by the immunocompromised, and then see how much benefit and risk it conveys.
merylnass:Dr. Shah from Maine points out the issue of having to stock so many similar vaccines. It means the clinics throw more away; it costs the clinics more; the staff make more mistakes.
merylnass:Grace Lee likes the new vaccine for the immunocompromised for 12,000 Americans. But the mfrs will not produce vaccines for such a small population and will rely on general use, once recommended.
merylnass:Helen Keipp Talbot points out that the models ignore post-hospital disability and this would make the vaccines more useful.
merylnass:Sarah Long points out that the PCV7 in kids helped adults, but the PCV13, which came out later, did not--so the PCV20 may not either. I like her.
merylnass:I suspect the cost of the PCV20, based on Prevnar costs, is likely to be $250-300 per dose.
merylnass:The last speaker implies they should approve it now cause it takes a year to get the insurers to cover it, so let's get ahead of that
merylnass:This speaker says they should put pressure on Pharma for rational pricing. Duh. Lowered price could make cost-effectiveness better.
merylnass:Kudos to the pharmacist whorepresents a national organization for pointing out pharmacists generally do not have access to the medical record and cannot necessarily make good decisions and provide good advice to patients
merylnass:Current speaker said she got the PCV20 and insurance would not pay and she was charged $247 for a dose out of pocket--because it was not recommended by ACIP for a year, I guess.
merylnass:Be aware how arbitrary the decisions this committee will make will be, based on little more than speculation
merylnass:The staff are asked to provide cleaner questions for a vote. Because the members are confused by what is being asked of them. Dr. Kobayashi wants to clarify. It seems there are never too many vaccines and never too many permutations for how they can be used.
merylnass:I am avoiding all this minutiae about changes to existing recommendations. But note the vagueness of the adverse event information. You don't learn what they are. And you get a meaningless statement that most of the reactions were mild or moderate and not severe. Well, that is true of every vaccine I know of. It conveys no infomration. It hides relevant information. It fills in the space where real data about adverse events should be specified with their rates, but are not.
merylnass:Ten minute break till 11:35. We are already nearly an hour behind.
merylnass:Chikungunya vaccine now. Votes on the pneumococcal vaccines later.
merylnass:No chik vaccine has ever been licensed, but I am aware of attempts to make one for probably 30 years. Which may mean there are intrinsic problems with making a vaccine for this condition.
merylnass:The US government's DOD has long been interested in this virus, though there were no US outbreaks until 2013.
merylnass:Aedes aegypti and albopictus daytime dusk-dawn mosquitos carry it, but there can be vertical transmission, needlestick, and even airborne in labs.
merylnass:Note that we are not told how frequent the serious complications may be. Not good in pregnancy, it seems. Usually it lasts 7-10 days but there is occasional prolonged symptomatology
merylnass:It seems the studies are not very helpful. Bug repellants are the preventive.
merylnass:Valneva and Emergent BioSolutions, the crooked anthrax vaccine mfr that threw away the ingredients for 400 million doses of cOVID vaccines due to contamination is developing one vaccine--stay away from it. They also now own a cholera and a typhoid vaccine. Their typhoid vaccine made one of my patients fairly ill 2 years ago.
merylnass:There is also a Merck and an Indian candidate vaccine, both sponsored by CEPI (started by Bill Gates)
merylnass:It seems there is an interesting business plan: bring a tropical disease to the US that was never seen there before, then bring in a new vaccine for the disease. This has happened with Dengue and Dengvaxia
merylnass:They say that human challenge studies can be justified. This new unethical way of studying a vaccine is apparently now joining the ranks of acceptable clinical science.
merylnass:It looks like the Animal Rule is being invoked to get the Valneva version licensed under accelerated approval. Dr. Chen asks who is really at risk of a complicated course?
merylnass:Remember that less than 100 Americans per year get this infection, though the numbers could rise. Dr. Hills sounds stressed, as if she does not want to answer, probably due to lack of data. Though mother-fetus transmission can be devastating.
merylnass:Dr. Brooks asks how outbreaks get started--especially in the US, when the disease is so rare?
merylnass:She dodges the question of how it gets to a place where it has not previously been seen.
merylnass:Dr. Dubischar from Valneva now presents on their candidate vaccine. It is live with a genetic deletion of 60 amino acids in one protein.
merylnass:Neutralizing antibodies--human sera injected into monkeys who were then challenged with the real virus. The injected primates had no fever and most lacked live replicating virus. Some did have virus but lower titers. Valneva believes they have identified a dose that will provide sterilizing immunity, i.e., no virus growing in patients after vaccination and exposure.
merylnass:Some monkeys did grow virus, which is what is telling Valneva how large a dose to use to prevent virus in the vaccinated and exposed.
merylnass:Medium dose (3 x 10 to the 4th) viral particles is being used.
merylnass:They were allowed to skip Phase 2 by assuming that the booster dose acted as a challenge study.
merylnass:Solicited adverse events were only collected for 10 days--nice work if you can get it! Great way to avoid finding serious side effects. Vaccinees followed up to 6 months for unsolicited side effects.
merylnass:Odd that neutralizing antibodies were identical in those over and under 65 years.
merylnass:The immunogenicity seems remarkable, with antibodies in over 96% at 6 months. The side effect profiles are not good however.
merylnass:50% had systemic AEs, some severe fevers
merylnass:17% of recipients got joint pain vs 5% in placebo. In 3,000 subjects, one case of SIADH occurred 10 days after his shot. One developed muscle pain requiring a 5 day hospitalization. Serious AEs in 1 in 1500 recipients--but more may have been overlooked because of the short duration of active surveillance.
merylnass:73% had any AE. Seems like you will be better off accepting a very low risk of getting chikungunya than taking a chance with this vaccine.
merylnass:Why is the ACIP looking at these vaccines before they are licensed? This makes sure there is a lot less data and less give and take because they don't have the info that would have been presented to FDA.
merylnass:While pregnant women are the ones who would potentially get the most benefit, the company is clearly scared to death to test the vaccine in them, as it is a live vaccine and will likely cause intrauterine infection.
merylnass:0.3% had joint pain severe enough to interfere with daily activities. Mean duration 8 days.
merylnass:Dr. Sanchez asked about the joint pains--any arthritis? And pregnancy?
merylnass:OOps. She had to admit women did get pregnant during the study and they had miscarriages and normal births and she claims it was what would be expected generally. If so, she would have presented those data as evidence of safety. The fact that she failed to present them initially suggests there is a potential problem. Dr. Sanchez asked if the fetus got infected? She says we didn't look. That is the clincher.
merylnass:Now the speaker was instructed by a committee member to do pregnancy studies in monkeys.
merylnass:Now CDC presents again on the vax. 100 of 462 subjects were dropped due to protocol deviations--this is a high dropout rate.
merylnass:Most stopped coming back. Hmmm.
merylnass: 2% had a severe adverse event. That is VERY HIGH, especially when the placebo group only had 0.1%
merylnass: 2% of the subjects had joint pain for over 15 days. Sounds like we are normalizing prolonged side effects from vaccines now
merylnass:The data have only had a preliminary review--so why is this being presented to ACIP when it seems there is no good reason to license this, as there is almost no chikungunya disease in the US and the side effect profile is poor; furthermore, there were no data presented to convince us the disease is a serious problem.
merylnass:Except in pregnancy, for whom the vaccine is probably too dangerous.
merylnass:Someone asked about simultaneous live virus vaccines being given. I will say that in literature 20 years ago, giving lives vaccines together was said to reduce the immunity induced.
merylnass:So it was NOT RECOMMENDED to give live vaccines together. But that was when there was still a modicum of real science. I am not surprised Valneva refused to answer.
merylnass:Hills from CDC also refused to take up the question.
merylnass:Severe immunocompromise will be a contraindication to this vaccine
merylnass:What a cluster. A dangerous vaccine for which limited data are made available for what is usually a minor disease that almost does not exist in the US.
merylnass:Break till 1:30
merylnass:Next up, COVID VACCINES
merylnass:From a 2007 Senate report headed by Tom Coburn https://www.cbsnews.com/htdocs/pdf/cdc_off_center.pdf
CDC’s $106 million Thomas R. Harkin Global Communications (and Visitor) Center • CDC’s new $109.8 million Arlen Specter Headquarters and Emergency Operations Center has $10 million in furniture • CDC’s $200,000 fitness center includes $30,000 saunas and rotating light shows • CDC’s new Hawaii office announced by Hawaii Senator who oversees CDC funds
merylnass:No wonder they don't manage to do competent reviews of vaccines; they are spending too much time in the saunas or at the Hawaii office. Remember that these amounts are 2006 dollars
merylnass:Announcement: TODAY the Novavax was approved by FDA for use as a booster in addition to an initial vaccine for adults. Despite the ACIP meeting today,the members were not asked to approve this rollout, and Rochelle Walensky has already approved it for adults. Strange omission.
merylnass:The lesson is that the fed agencies are in a RUSH to get all this investigational garbage into arms as fast as possible before the entire program explodes.
merylnass:7 days ago the FDA granted an authorization for moderna and Pfizer boosters for kids 5 years and up.
merylnass:Ellington slipped and just said "pregnant women" and then slipped in the word people a few words later.
merylnass:The data being presented here come from a BMJ article published in 2020--when there were much more severe variants and much fewer treatments available, so it is not surprising that pregnant WOMEN had significantly worse morbidity and mortality than non-pregnant women. Pregnancy itself turns down the immune system
merylnass:While the intent of the presentation is to scare us regarding 1-6 month old babies, in fact COVID was present in only 0.5% of babies who died over the pandemic.
merylnass:This presentation uses CDC's VSD data to assess risk of vaccination in pregnancy from the earlier vaccines. This database in earlier studies never showed a problem with the mRNA vaccines. Finally it did so re myocarditis, but has failed to find any other problems. I suspect there is a crooked method of analysis. CDC never lets us know how these numbers were derived, how they adjust them and how they analyze the raw data.
merylnass:As expected, these data claim you supposedly actually benefit slightly in terms of miscarriage from the vaccine.
merylnass:And they are assiduously studying the data and stay tuned for more happy news
merylnass:I have screenshot all the people involved in these studies for future reference so we can get them discussing these studies with their hands on a bible
merylnass:The V-safe data shows less than half the expected miscarriages in vaccinated moms. Do you believe these data? Of course not--it is impossible to have such low rates. That proves there is a problem with the study.
merylnass:Furthermore, the claim is being made that comparing vaccinated pregnant women to vaccinated pregnant women who got covid, the covid patients did not have a worse fetal outcome. How likely is that?
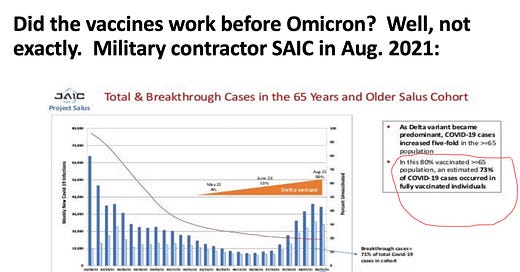
merylnass:Now Fleming-Dutra presents and gives the commercial that everyone should stay up to date with the vaccine and pregant and breastfeeding women should especially get it. What? Her data claim the vaccine worked wonderfully for Delta. But here is some DOD contractor data on Delta in the vaccinated elders—about 10% benefit
merylnass:Now the bivalent commercial. But they will show no bivalent data.
merylnass:Note that almost every presenter is from CDC and they have had a team work on the presentations so every word has been crafted. That is probably why you aren't allowed to see the presenters, because they are reading their script.
merylnass:These data on omicron and delta carefully avoid any numbers after 4 months post vaccination, when efficacy goes to zero and worse
merylnass:But other charts that followed people to 6 months and beyond show how useless they become, and then worse than useless.
merylnass:I am going to post this speaker's diagram on efficacy on my substack during the next break, so you can see how different the longer-term results are, and how the vaccine didn't work well for delta either.
merylnass:No infant protection when pregnant women were vaccinated before 20 weeks!!!!
merylnass:This woman is working hard to get up the CDC ladder by throwing all women and children under the bus.
merylnass:Thanks Dr. Sanchez for asking about monoclonal antibodies in pregnancy. The briefer does not answer the question, turning it into a vaccine question. Someone else says we have not been collecting data on the use of other therapies.
merylnass:No one wants to talk about the monoclonal antibodies in pregnancy, which is relevant to my legal case, as the state has claimed I should have used monoclonals in a pregnant woman.
merylnass:But they are experimental while the drugs I used are fully licensed and effective.
merylnass:More missing data for Sarah Long's question.
merylnass:Some of the CDC people on MISC could not be here today. Let me point out that these slackers are working from home, and they could have been available for questions had CDC wanted them available.
merylnass:Sarah Long asks about what symptoms the hospitalized, covid-infected babies had...and 3 different CDC briefers refused to answer. Is that because they only had sniffles and were hospitalized for something else? Were they asymptomatic and tested positive incidentally?
merylnass:Great, someone points out that 77% of women are not vaxxed, though this telephone survey data says they are. In the data I have seen, about 1/3 of pregnant women took a covid shot, not 3/4. In this survey, CDC phones people and asks if they are vaccinated--and many people simply tell CDC they are vaxxed when they are not. If memory serves, once CDC used this NHANES survey to estimate that more americans had gotten a flu shot than the number of flu shots that had been manufactured distributed in the US.
merylnass:The above paragraph is about pregnant women and now the members are saying "I find these data sketchy!"
merylnass:Fleming-Dutra: vaccine was less effective with omicron. She did not answer the question asked, which was what is the ideal time to vaccinate in pregnancyc. I saw a slide go by that said the baby got no benefit during the first 20 weeks. Now these CDC apparatchiks want women to get 1 vaccination during the first 20 weeks and another dose during the second half of the pregnancy. I do not have a name for these evil doctors.
merylnass:They can still surprise me by how awful their suggestions are and how they just keep getting worse. Now the ACOG rep is calling them "pregnant individuals." She is an obstetrician and she does not know the gender of her patients?
merylnass:Sara Oliver, oh no. 6month-4 year olds had the highest hospitalization rate she says. But a previous speaker said the 0-6 month olds had the highest rate. How fun that they cannot keep the lies straight.
merylnass:Sara fails to tell us where these data come from and what all the numbers were. She claims incorrectly that myocarditis is 1.8-5.6x higher after infection than after the shot.
merylnass:Sara says we are just discussing the current recommendations. Is she saying they are not going to put the vaxxes on the childhood schedule? Who knows what the forked tongue doctors of the Centers for Dissimulation, Craftiness and Prevarication really mean
merylnass:She is reminding us to give boosters to all kids aged 5 and up
merylnass:For immunocompromised kids, they want 3 initial doses. May I point out that although the CDC encourages mix and match mRNA vaccines, a very important study from 4 Nordic countries showed that by giving a Moderna after a Pfizer in children, you increased the likelihood of myocarditis by a factor of two compared to giving both doses of Moderna. Furthermore, many countries in Europe do not allow you to give the Moderna vaccine to children and younger adults because of this. So CDC should never have encouraged mixing the vaccines. Here is some info I put togehter in August about this:
In 7 European countries, people below certain ages were not recommended to get Moderna shots, but as of June babies as young as 6 months can get Moderna injections.
Sweden—not under 30 https://www.wilx.com/2021/10/07/some-european-countries-suspend-moderna-shots-those-30-under/
Norway—not under 30 "
Denmark—not under 18 "
Finland—not under 30--https://www.cnbc.com/2021/10/08/nordic-countries-are-restricting-the-use-of-modernas-covid-vaccine.html
Iceland: "Iceland is using the vaccine almost exclusively as a booster for those 60 years and older, and advising men aged 18-39 against receiving Moderna’s vaccine (here)." https://www.reuters.com/article/factcheck-europe-moderna/fact-check-some-european-countries-halted-moderna-covid-19-vaccines-for-young-people-idUSL1N2RE22K
Germany —not under 30. https://www.forbes.com/sites/roberthart/2021/11/10/germany-france-restrict-modernas-covid-vaccine-for-under-30s-over-rare-heart-risk-despite-surging-cases/
France—not under 30. https://www.forbes.com/sites/roberthart/2021/11/10/germany-france-restrict-modernas-covid-vaccine-for-under-30s-over-rare-heart-risk-despite-surging-cases/
merylnass:Now she says that incorporating covid vaxxes in the immunization schedule and the vaccines for children program (govt paid) is a step toward including them into a regular routine schedule, which will happen once the vaccines are commercially sold, and can have the 75 cent excise tax added that is necessary to be placed on the childhood schedule.
merylnass:Jeanne Santoli notes the youngest kids got authorized 120 days ago. It appears this is getting the vaccines ready to become normal products for kids that are not EUA products. Commercialized. At that point vaccines can be ordered through the VFC, which is a govt program that supplies free vaccines for about 55% of US children. In other words, what the USG is doing si worming the vaccines slowly into the childhood schedule by taking baby steps toward that, and this is one baby step.
merylnass:A member is asking for clarity on whether this is going to put it on the schedule and mandate it. Jose Romero says they are not putting it on the childhood schedule yet.
merylnass:But now he is backpedalling--"CDC does not make state recommendations for vaccines"--yet CDC knows that many states have adopted the CDC recs as their own.
merylnass:A good question: will the vaccine still be part of the CICP or moved to the VICP? Melinda Wharton says the vaccines stay in the CICP.
merylnass:Things are ahead of schedule!
merylnass: Comments. I love David Wiseman, who point out that CDC is recommending use in pregnancy that is much broader than the manufacturers' own claims.
merylnass:I noticed when the ex-officio members were listed today that 2 (TWO) of them had been commenters in favor of the vaccine program at a recent ACIP meeting (Stinchfield and Sean O'Leary). Isn't it remarkable that an ex officio member of the ACIP had to grab a slot at the public comment time to laud the vaccines? Was CDC desperate to fill those slots in order to keep those of us with a different opinion out?
merylnass:The current speaker, a father, is great. COVID is not a childhood disease. It does not affect transmission. The benefit wanes quickly and new variants evade them. He calls out the multiple fraudulent efforts to fool us about the disease and the vaccine.
merylnass:Thayer Phillips is a spokesperson for 'seniors speak out.' Does CDC fund this organization? "Now is the time we must prepare ourselves to protect those most vulnerable."
merylnass:He is boosting for the new expensive PCV vaccines for elders--which are currently available but will cost ya.
merylnass:Now we have a similar spokesperson from another older person organization. He too is a PCV booster. Maybe one was paid by Merck and the other by Pfizer. Now the National Grange is represented and it too is here to boost the new PCV vaccines. Incredible. I guess at $247 per dose the mfrs can afford to purchase lots of elders to lecture to CDC, and fill up spots that might otherwise go to discussing COVID vaccines.
merylnass:There were only 6 speakers. Now we go to the PCV vote and the VFC vote.
merylnass:Finally we get to see some faces as they vote and 2 female members of the ACIP are masked. 15 yeses zero nos for the vaccines for children vote, which as I said is only a baby step toward the childhood mandates.
merylnass:Now for the totally confusing PCV votes, because there are 3 adult PCV vaccines and another one coming soon, and they all have a different collection of serotypes...so how and when do you mix an match them, when there is very little data to inform us about the answer?
merylnass:And the answer is that it probably does not matter and most likely most of the members would not be able to explain what precisely they are voting on right now. So I predict you will get unanimous yeses on all 3 questions.
merylnass:You know what? I think that none of our CHD watchers care about the outcome. Maybe we can all simply shut down this meeting before we are all braindead. I told you no one understood these votes.
merylnass:I forgot, they have to talk about polio vaccines too, and that might be important. WHO just collected several billion dollars over the past few days to roll out a new polio program.
merylnass:They decided not to vote! CDC will have to make the questions simpler and clearer--or if CDC doesn't want to do that, they will have to figure it out later.
merylnass:Polio vaccine: 1961-1999 used primarily live vaccine. In 2000 ACIP switched to inactivated vaccine because all polio cases were due to vaccine in the US.
merylnass:Numerous vaccine strain outbreaks are being detected.
merylnass:While only 'wild type' type 1 polio appears in the world, other types of polio exist due to vaccine strains 2 and 3.
merylnass:What is not being said is that it has long been known that some people shed the poliovirus after the injection for a lifetime.
merylnass:So there will be polio virus in wastewater over much of the world.
merylnass:Polio is shed in the stool.
merylnass:Interestingly, fractional injected polio vaccine at 20% the normal dose, given intradermally, is being used in some countries outside the US.
merylnass:This must be where some bright bulb got the idea to dilute the monkeypox vaccine.
merylnass:What I need to emphasize is that the NYC case does not mean much, nor does the wastewater analysis showing polio in multiple water systems. Because there has probably been vaccine strain polio in wastewater for many years, and probably many people have been exposed over the years. But only about 1 in 1000 people exposed actually develops a case of polio.
merylnass:It is probably more important to keep kids out of dirty water, and avoid contamination of drinking water, which can happen when sewer lines rupture, etc.
merylnass:Dr. Sanchez asks about earlier data on wastewater. The CDC person says we are following some guidelines that tell us only to look at water around cases. DUH. Don't look don't tell, right? Of course they do have this data, because we found from COVID that Europeans were able to test water from the past year for evidence of SARS-CoV-2 in the year before the pandemic started.
merylnass:They kept wastewater samples. But CDC does not want to go there and admit that polio is being shed in multiple locations.
merylnass:Dooling said they tested water in CT and NJ and the water tested was negative.
merylnass:Well, for once the CDC did not tell everyone to run out and get a polio booster! Maybe there is a shortage. BTW, the injected vaccine used in the US is not nearly as effective as the live vaccine that can revert to virulence. I guess they don't want people to get boosted and then come down with polio, showing the vaccine did not work as well as claimed.
merylnass:Till 8:30 am tomorrow. G'nite.



Thank you ■ it was a luxury to have your live blog today; enjoyed your honest observation and discernment in the details during [t]heir meeting. Meryl you are an example and I deeply appreciate your bravery in the storm of tyranny.
Meryl Nass, we appreciate you.