FDA uses army of researchers to conclude that maybe pulmonary emboli are linked to COVID vaccines, but 13 other diagnoses are not
My quick and dirty analysis of FDA's BS paper
How many USG-employed electricians does it take to screw in a light bulb? Ask the FDA’s team of epidemiologists at its Center for Biologics. I count 16 authors and 25 additional support staff who contributed to this exercise. And all they did was see whether people on medicare who were vaccinated had more episodes of 14 illnesses than were expected post vaccination. That is not hard. They did not even look for very long: only 28 days after a shot for 7 diagnoses, and 42 days for the other 7 diagnoses.
https://www.sciencedirect.com/science/article/pii/S0264410X22014931
They needed 4 assistants to help with writing. Why? They used a Martin Kulldorff statistical package; does that keep him inside the corral, to an extent, such that he still says elders should be vaccinated?
Note also that the paper was initially rejected, and it took 6 months to be resubmitted. Why did it take so long? With 41 people working on it, no less?
There were over 25 million medicare enrollees aged over 65 in this study, yet only 32.2 million shots given, or 1.27 shots per person enrolled, suggesting that many elders only got one shot (only under 600,000 medicare enrollees received a single dose J and J shot) and/or many refused.
Yet according to the NY Times, 93% of Americans over 65 have been fully vaccinated. So it seems either FDA used a highly unrepresentative sample—yet the sample was supposed to be composed of all vaccinated medicare enrollees who had been on medicare at least a year, so if the authors were being honest it was totally representative. Or the NY Times, which gets its data from CDC, is very wrong about the percentage of vaccinated elders.
Either the FDA’s data or the CDC’s data (or perhaps both) are wrong.
Did CDC just carve out an excuse for providing inflated data i.e., “may overestimate first dose coverage among adults.”
The first anyone heard of FDA’s medicare database analysis was July 12, 2021. This is what it said:
Initial Results of Near Real-Time Safety Monitoring of COVID-19 Vaccines in Persons Aged 65 Years and Older
July 12, 2021
FDA has routinely been using screening methods to monitor the safety of COVID-19 vaccines and to evaluate potential adverse events of interest (AEI) related to these vaccines. One of these methods, called near real-time surveillance, detected four potential AEIs in the Medicare healthcare claims database of persons aged 65 years and older who had received the Pfizer/BioNTech COVID-19 vaccine. The four potential AEI are pulmonary embolism, acute myocardial infarction, immune thrombocytopenia, and disseminated intravascular coagulation. The screening methods have not identified these AEI after vaccination in persons 65 years and older who received the two other authorized COVID-19 vaccines…
FDA continues to closely monitor the safety of the COVID-19 vaccines and will further investigate these findings by conducting more rigorous epidemiological studies. FDA will share further updates and information with the public as they become available.
The paper I am discussing is the first update. Note that the initial analysis was published to FDA’s website by 7 months after vaccinations started. This update, on a very critical topic, took an additional 16 months.
The supplementary tables provide the numbers of recipients for dose 1 and dose 2, and consistent with other CDC/NYT data, there was a 10-12% dropout rate after the first shot.
31 million doses were said to have been given, but the study only considered under 11 million first doses and 9.3 million second doses. So what happened to the other 10 million doses? Were those subjects dropped? There is not enough detail provided to understand what the researchers actually did with the data. The detail should have been provided in the supplementary materials, but was not.
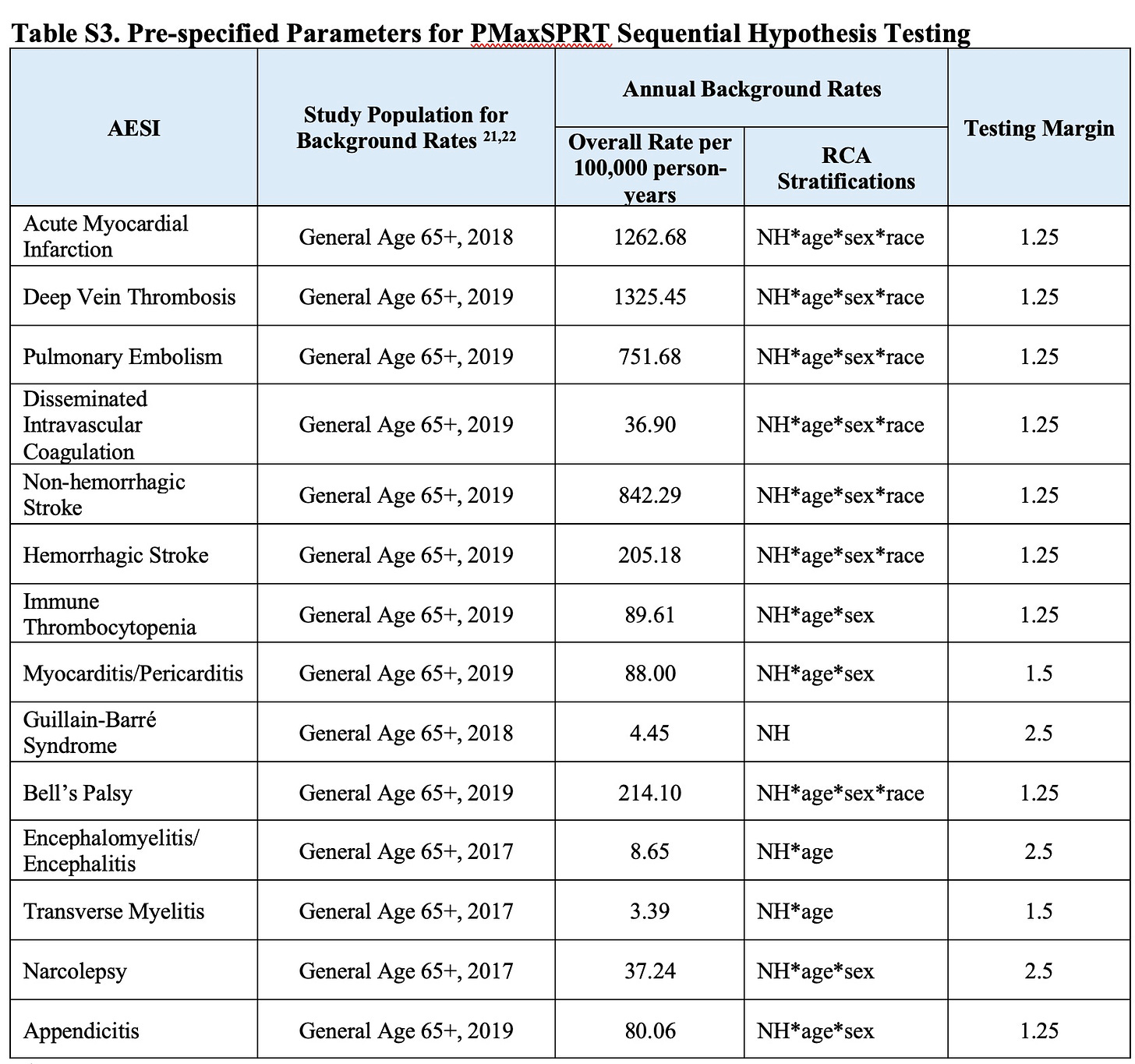
Furthermore, the study authors used a set of numbers as their ‘expected’ rate of occurrence of each diagnosis, but we can’t verify these numbers, nor whether they are appropriate for the ages included in the study. The footnotes listed in the chart below (21,22) do not link to any literature.
If the ’expected’ comparison numbers are too high, they will cancel out a true rise in cases. Had I done this study, I might have used medicare recipients who were not vaccinated as an additional comparator group.
Here is the laughable way the authors describe their ‘method:’ “Claims-based outcome algorithms were developed based on literature review and in consultation with clinical experts [8].”
Well, I read footnote 8 and discovered that this is precisely where the authors got their ‘expected’ rates: all their rates are within a percentage point of those listed in reference 8 (in an analysis of the medicare database) and some of the numbers are identical. Not sure why they beat around the bush about this.
Below are the baseline comparators FDA chose to use:
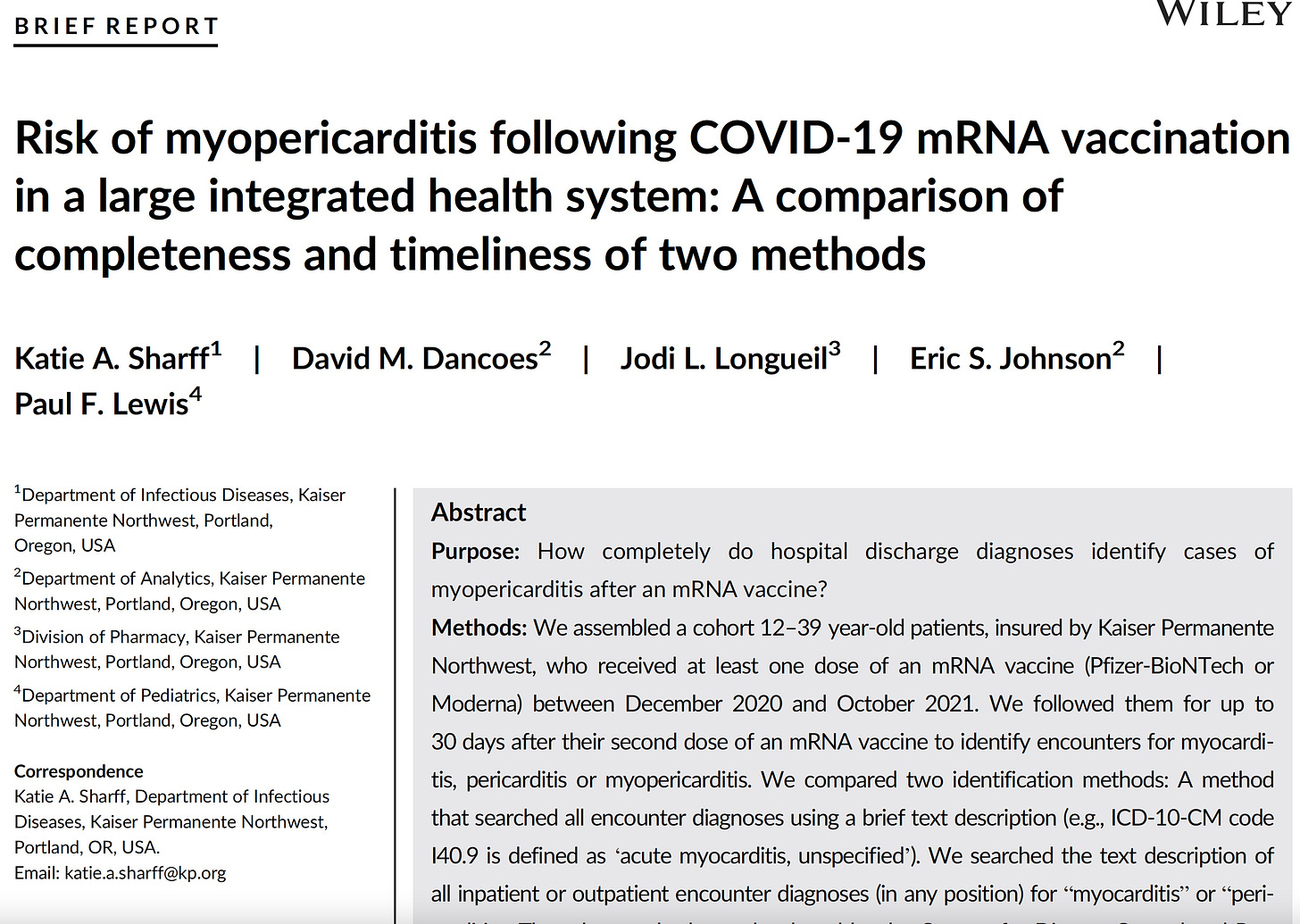
Finally, we are not told the diagnosis codes uses to collect all the cases of disease. If the authors failed to include all the relevant codes, cases will be missed. This is what happened when the CDC’s consultant, Nicola Klein, counted the myocarditis cases for CDC and found no elevation, as discovered by Sharff et al.
Bottom line: This is a very poorly-described study of unknown methodology, whose data are questionable, and which purports to show a tiny increase in pulmonary emboli after COVID vaccinations but no rise in cases of 13 other conditions post-vaccination. The conditions for which it claims no increase after vaccination include Bell’s Palsy, heart attacks and myocarditis. Its findings defy common sense.
We should furthermore ask why the rate of vaccination in this database of over 25 million medicare recipients is so much less than what CDC claims.
41 USG employees and consultants were unable to screw in this light bulb and provide a coherent analysis from the medicare database of post-vaccine injuries. Why?








An army of researchers? Give me a break. The CDC is one of the world's biggest jokes on a par with WHO and the UN, FDA, NIH, IRS, SEC, FBI, AMA, etc, etc, on and on.
FDA liars club