
Lawrence O. Gostin and Daniel Troy (former FDA Chief Counsel) weigh in on Kennedy nomination. Let's put them under a microscope.
Both Gostin and Troy got rich through paying homage to the status quo and big business as lawyers, while harming consumers. No wonder they are still shilling.
https://www.wsj.com/public/resources/documents/cJ2DGNAaDPf9KVD6KykC-WSJNewsPaper-11-18-2024.pdf
Let’s look at the Prolific Professor Gostin, who seems to have an opinion about everything. Especially Rock Solid Science, a subject for which he has no credentials. https://www.scientificamerican.com/article/vaccine-mandates-are-lawful-effective-and-based-on-rock-solid-science/. Note that the Editor-in-Chief of Scientific American resigned a few days ago after publishing a nasty public rant, after turning the magazine into a Democrat party screed:
Just look at the title of what she published as the mandates were going into effect. Gostin was reading (or writing) Fauci’s script. Remember, Gostin wrote the state laws that could force vaccinations and quarantines on us. Now he assures us they are legal.
The U.S. has reached a worrying plateau in its COVID-19 vaccination coverage, with just half of the population fully vaccinated. This coincides with pandemic fatigue, or weak compliance with COVID-19 risk-mitigation measures such as masking and distancing, and the highly infectious Delta variant, which accounts for more than 83 percent of infections. We’re at an inflection point in the pandemic, with coronavirus infections soaring by about 140 percent in the past two weeks.
Reflecting deep concern over lagging vaccinations, the Biden administration recently mandated vaccinations for all federal workers and contractors (with masking and regular testing as an alternative option), while the Department of Veterans Affairs issued mandates for all frontline health workers at its facilities. President Joe Biden also ordered the military to move toward compulsory vaccinations.
California, New York City and New York State are leading the way in requiring that government workers get vaccinated or else submit to weekly testing. And New York City just announced its “Key to NYC Pass,” requiring proof of vaccination for access to most indoor activities, including gyms, restaurants and performances, beginning on September 13—the first policy of its kind in the U.S. Meanwhile more hospitals and long-term care facilities are implementing mandates following a joint statement from 100 medical and nursing groups urging compulsory vaccinations. And when schools of higher education return in the fall, nearly 600 colleges and universities will require vaccination. That’s true in the private sector as well as a rising tide of businesses—including Uber, Facebook, Google, Netflix and Delta Air Lines—mandate vaccinations for workers.
Are these mandates lawful and ethical? The short answer is emphatically yes. And there is strong behavioral science evidence that mandates will be highly effective.
The Lawfulness of COVID-19 Mandates
Businesses: Both the Department of Justice and the Equal Employment Opportunity Commission (EEOC) have ruled that businesses may lawfully require workers to get a COVID-19 vaccine as a condition of coming to the workplace. However, businesses must grant legitimate medical or religious exemptions. The only major court ruling to date upheld Houston Methodist Hospital’s COVID-19 vaccine mandate. The private sector has wide discretion in setting conditions for workers and customers, and businesses have a legal and ethical duty to keep the workplace safe.
Currently all COVID-19 vaccines are administered under an emergency use authorization (EUA) and are not fully licensed. The DOJ and EEOC specifically stated that employer mandates may occur even under an EUA. [But there was no statute in place to support their claims, and this was a change in policy—Nass] The U.S. is already acting as if the vaccines were fully approved, with public health agencies recommending that all eligible Americans get a shot. In any event, the Food and Drug Administration recently announced it is expediting full licensure of the Pfizer vaccine, which is likely to occur by September, with other vaccines not far behind. When the FDA does approve COVID-19 vaccines, more businesses are likely to require immunizations.
Federal and state governments: States have long had the constitutional authority to mandate vaccinations, which the Supreme Court has upheld twice, first in 1905 and then in 1922. The federal government, however, has limited power to mandate vaccines. It can only require them to prevent transmission of a dangerous infectious disease across state lines or international borders. The federal government has never sought to require nationwide vaccinations, and the courts probably would not allow it. To date, all state government mandates have been for fully approved vaccines. Thus, it is likely cities and states would wait to mandate COVID-19 vaccines until they are fully licensed. But when governments act as employers, they would be in a similar legal position as businesses. Thus, federal and state worker COVID-19 vaccine mandates are fully lawful even under an EUA.
K–12 schools: Every state and Washington, D.C., require routine vaccinations, such as for measles, mumps and rubella, as a condition of school attendance. While state mandates vary, all are based on a long list of childhood vaccinations recommended by the Centers for Disease Control and Prevention. In 1922 the Supreme Court upheld school mandates, and all courts have recognized the states’ authority to mandate fully approved vaccines. Schools must offer medical exemptions for those who may be harmed by vaccines, but they do not have to grant religious or conscientious objections. Six states offer neither personal nor religious exemptions for school mandates, a move that the courts have upheld. Because K–12 COVID-19 mandates would be issued directly by the government, they are likely to require full FDA licensure. Further, the FDA’s EUA for Pfizer’s COVID vaccine currently applies only to people aged 12 years and older, and the other two COVID vaccine EUAs are only for adults. Clinical trials for children younger than 12 years old are currently underway but may not report full results until late this year or early 2022.
Institutions of higher education (IHEs): In most respects, IHEs are in a similar position to businesses. IHEs have long required vaccinations of students—for human papillomavirus, meningococcus and influenza, for example. A federal court has already ruled that Indiana University’s COVID-19 mandate is a reasonable, science-based measure to ensure student health and safety. And the U.S. Court of Appeals for the Seventh Circuit just affirmed that Indiana University’s mandate was lawful. IHEs act in loco parentis and thus have a duty to care for the safety and security of students on campus. While most IHE COVID-19 mandates apply to students, many also apply to faculty and staff.
Antivaccination Laws
While there is a rising tide of governments, businesses and IHEs mandating COVID-19 vaccines, some states are actually seeking to restrict vaccine mandates. More than a dozen states have enacted laws prohibiting mandatory COVID-19 vaccinations or even “proof of vaccination” (so-called vaccine passports). Many more have bills pending. A number of governors have issued executive orders prohibiting COVID-19 vaccine mandates or vaccine passports. These edicts restrict private businesses, schools and IHEs from asking for proof of vaccination. COVID-19 has been politicized like no virus before. From AIDS and tuberculosis to Zika, influenza and Ebola, states previously acted to enhance public health powers. A multitude of states are doing the opposite during this pandemic, tying the hands of health officials to act quickly and decisively. That will not only make it harder to fight COVID-19 but also the next health crisis.
States have wide powers to regulate businesses, so even if these laws and executive orders are harmful, they still may be lawful. The exception may be states’ attempts to regulate businesses that operate in interstate or international commerce. There is litigation regarding Florida’s attempt to ban the cruise ship industry from requiring passengers and crew to show their vaccination status. Recently a federal court of appeals unanimously voted against a CDC order regulating cruise ships, which Florida had challenged. It was a bizarre case because the decision reversed the appeals panel’s earlier 2-1 ruling, which temporarily blocked a lower court’s decision to uphold Florida’s ban, and the appeals court has yet to give its reasoning for the change. The Constitution is quite clear that Congress holds the sole power to regulate interstate and international commerce.
Are Vaccine Mandates Effective?
There is considerable behavioral scientific data that vaccine mandates are effective. That includes both “hard” mandates (required vaccinations for school or workplace attendance) and “soft” mandates (the choice to vaccinate or undergo regular testing and indoor masking). Hospitals that have required influenza vaccinations have achieved and maintained far higher coverage than those that make it voluntary. At the same time, K–12 school and IHE mandates have given the U.S. high vaccination rates.
Many business and educational mandates fall into the “hard” category—that is, students or workers cannot attend classes or the workplace unless they are fully vaccinated. “Soft” mandates “nudge” people to get vaccinated. When getting a vaccine is the “easier” or “default” option, most opt for the jab. Thus, when given the choice between getting a vaccine or having to undergo one to two SARS-CoV-2 tests weekly and masking up, most people will eventually roll up their sleeves. How do we know? Well, in states that have wide and easy exemptions for childhood vaccines, a significant number of parents opt out. But if a state makes getting the exemption hard, such as requiring a written declaration, a doctor’s certificate or attending vaccine literacy classes, vaccine hesitancy melts away. The same will happen for COVID-19 vaccinations.
Are COVID-19 Vaccine Mandates Ethical?
People cite bodily integrity, personal liberty and freedom as the most common justifications for refusing vaccines. But these arguments don’t hold water. It’s true that everyone has the right to refuse a medical intervention for their own good. But vaccines not only protect the person vaccinated but also that person’s family, neighbors, and classmates or co-workers. No one has the right to go into a crowded classroom or workplace unmasked and unvaccinated. Vaccine mandates do not unethically discriminate. Discrimination is wrong when it is based on irrational reasons or animus, such as discrimination based on race, gender or disability. But vaccine mandates are simply a tool, and they apply equally to everyone. They don’t impute blame or seek to shame the unvaccinated. They are intended only to keep the entire population safe.
But by August 6 Rochelle Walensky had told Wolf Blitzer “the vaccines no longer prevent transmission.”—Nass
Finally, requiring proof of vaccination does not violate a person’s privacy. Individuals are free to decline to give information about whether they received a shot, but if they do decline, they must expect reasonable consequences to ensure everyone’s health. Additionally, federal health information privacy rules apply principally to health care providers and not to businesses or schools. There is also a public health exception to privacy rules.
Requiring people to get a vaccine is part of the fabric of American history going back to the Revolutionary War. General George Washington compelled troops to get a smallpox variolation, saying it was necessary to safeguard soldiers and to win the war. And not only is mandating vaccination lawful, but it is also an ethical responsibility. For far too long, Americans have asked the question, “What entitlements do I have as a rights-bearing citizen?” It’s now time to ask, “What duties do I owe to my neighbors, my community and my country?” Getting a COVID-19 vaccine as the nation and the world are undergoing a historic health crisis is badly needed for the common good and mutual solidarity.
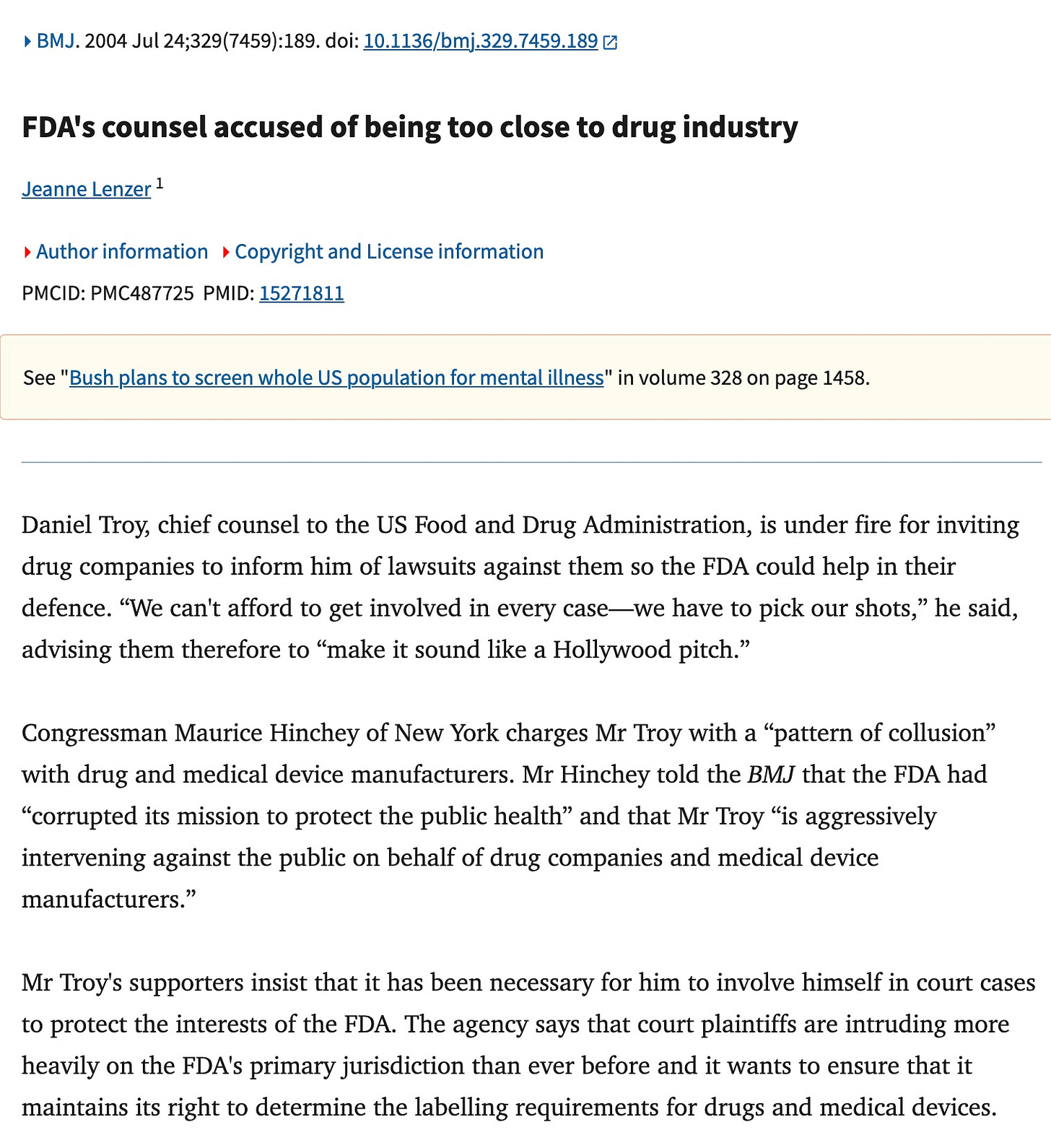
Let’s look at the controversial lobbyist Daniel Troy, who commented in the first article above. The first critical piece is from Mother Jones, the second from the BMJ. Mr. Troy is a piece of work. These are the paid mouthpieces trying to sink Bobby’s appointment. Know them for who they are. They will do anything to keep the wheels on their gravy train spinning.
When it comes to notorious Bush political appointees, Daniel Troy’s name doesn’t usually make the top-10 list, overshadowed as he is by more high profile cronies such as FEMA’s Michael Brown. But for three years in the president’s first term, Troy served as the chief counsel to the Food and Drug Administration (FDA), where he quietly advanced a legal revolution that is playing out in earnest in the U.S. Supreme Court this year. This revolution has the potential to affect the health and safety of the nation’s citizens for years to come, all while making Troy a rich man. In fact, his career is an illustration of how the Bush administration’s revolving door has allowed industry lawyers to radically reshape regulatory agencies to benefit the big businesses they once represented and then profit from those changes when they return to the private sector.
Back in 2001, when the president tapped Troy for the FDA, Bush made history for turning the general counsel’s job into a political appointment, when most of the previous occupants of the position had been civil servants. The appointment was a sign that the president intended to impose a political agenda on the agency. A former clerk to Robert Bork, Troy was a die-hard conservative who had spent most of his career suing the FDA on behalf of drug and tobacco companies over regulations those industries didn’t care for. Before going to the FDA, he had worked at the D.C. powerhouse law firm of Wiley, Rein & Fielding, where he represented a number of pharmaceutical companies, including Pfizer, which paid the firm more than $350,000 for Troy’s services the year he was appointed to the FDA. A year after the ban on working on matters involving former clients had passed, Troy quickly got to work helping out the drug companies who used to pay his fees.
Under Troy’s leadership, the FDA started showing up in state courts, offering briefs in civil cases on the side of drug companies being sued by people who’d been injured by dangerous prescription drugs and other medical devices. In an unprecedented move, agency lawyers argued that because those products had been approved by the FDA, such lawsuits were “preempted” by federal law and should be dismissed.
Troy’s FDA was promoting the idea that tort lawsuits in state court interfere with federal regulatory authority by letting judges and juries second-guess decisions over drug labeling and approval made by the experts in federal agencies. The drug companies were arguing, with the agency’s help, that FDA approval for a product should be the gold standard and that if companies meet that standard, they shouldn’t be subjected to the rules of 50 different states trying to impose their own health and safety standards through lawsuits.
Promoting preemption is a radical new policy for the FDA, which has long believed that, far from interfering with its mission, state tort lawsuits actually enhanced public safety by providing a financial incentive for companies to comply with federal regulations. Troy’s predecessor, Margaret Porter, a career official, wrote in 1997 that the agency had a long-standing policy against preemption, because “even the most thorough regulation of a product such as a critical medical device may fail to identify potential problems presented by the product…. Preemption of all such [tort liability] claims would result in the loss of a significant layer of consumer protection.”
The Bush administration has embraced preemption largely because it allows the White House to use its regulatory power to achieve what big businesses haven’t been able to do through legislation, which is immunize themselves from lawsuits over defective products. In recent years, federal agencies, dominated by industry insiders, have written preemption language into safety regulations, from rules issued by the Consumer Products Safety Commission governing mattress flammability to those introduced by the National Highway Traffic Safety Administration on seat-belt placement and automobile-roof strength. In fact, while the NHTSA was run by a former lawyer for Daimler Chrysler, it issued a roof-strength regulation that was so weak the only possible explanation for its issuance was that it was designed to help auto manufacturers head off lawsuits. Even the EPA has gotten into the act, once filing a brief on behalf of Dow chemical supporting its preemption defense in a case before the Supreme Court in 2005.
But no federal agency has pursued a preemption agenda as aggressively as the FDA did when Troy worked there. In 2003, he appeared at a conference of defense lawyers and in-house legal counsels from drug companies and publicly invited them to contact his office if they had a case the FDA could help them out with. “We can’t afford to get involved in every case,” he said, according to an affidavit written by someone who was there. “We have to pick our shots…so make it sound like a Hollywood pitch.”
During Troy’s tenure, between August 2001 and November 2004, the FDA participated in several private lawsuits, including one involving Pfizer, and argued that state courts and juries were illegally usurping federal regulatory power by trying to impose safety standards on drugs or medical devices approved by the FDA. The results of Troy’s intervention in the state court suits were mixed, but, to be sure, the FDA’s position bolstered the drug companies’ position.
Troy’s work at the FDA didn’t go unnoticed. In 2004, after he attracted some negative publicity for coming to the aid of his former client Pfizer in a lawsuit, Rep. Maurice Hinchey (D-N.Y.) attempted to defund his office. In November of that year, Troy retreated to private practice, where he continues to counsel companies regulated by the FDA. But before he left, he ensured that the preemption doctrine was firmly enshrined at the FDA in regulatory language covering drug labeling.
The “preemption preamble,” as it’s known, states that this rule preempts all state tort lawsuits against manufacturers who make drugs approved by the FDA. The regulation allows drug companies to invoke the FDA’s approval to beat back lawsuits without actually having to get the agency to enter the case. This is a hedge against future Democratic presidents who might want to institute a change of course. The preamble was inserted into the regulation without any public input. Since then, lawyers have repeatedly invoked it when defending companies against personal-injury lawsuits.
The courts have not swallowed Troy’s work whole hog. Several courts have rejected the preemption argument outright, and as a result the Supreme Court, which has not been especially sympathetic to injured plaintiffs, recently decided to clear up the confusion. Its most significant ruling arrived in late February in Riegel v. Medtronic. The case was filed by the family of Charles Riegel, who was injured when a balloon catheter burst during an angioplasty. He later died, and his wife sued the manufacturer, alleging that the catheter was defective.
The court ruled for the manufacturer in an 8-1 decision, siding with Troy, who filed an amicus brief on behalf of the medical-device industry arguing that because the device had received premarket approval from the FDA, private lawsuits in state court were preempted. The decision effectively wipes out pending litigation over everything from faulty pacemakers to defective insulin pumps. But the decision is a huge boon to Medtronic, a company whose conduct in the marketplace has hardly been stellar. Not only has it been accused of bribing surgeons to use its products, but the FDA has recalled a host of the company’s products. Now, people who have been injured by some of Medtronic’s products will have no way of forcing the company to pick up the tab.
Last week, the Supreme Court heard arguments in a second preemption case, Kent v. Warner-Lambert, which was filed by Michigan residents injured by the recalled diabetes drug Rezulin. Michigan bans lawsuits against drug and medical-device manufacturers for products approved by the FDA, but it has an exemption allowing suits to proceed if the manufacturer won approval of a product by engaging in fraud. Troy’s firm represents Warner-Lambert in the case. On behalf of the government, the U.S. solicitor general argued that even conning the FDA into approving a product wasn’t a good enough reason to allow a suit proceed. On Monday, the court upheld a Second Circuit court decision permitting the case to move forward.
But the decision was hardly a reason for plaintiffs and consumer advocates to rejoice. The only reason the defendants didn’t prevail was because Chief Justice John Roberts owns stock in Warner-Lambert’s parent company, Pfizer, and had to recuse himself. In all likelihood, he would have voted with the other conservatives in favor of the manufacturer. The 4-4 decision doesn’t bode well for the plaintiffs in another coming drug case that the justices decided to take up, much to the surprise of veteran court watchers. The plaintiff in Wyeth v. Levine is a musician who won a multimillion-dollar jury verdict after her arm was amputated due to an improperly administered drug. The drug company, Wyeth, is arguing that the verdict should be overturned because the FDA’s 2006 preemption preamble on drug labeling bans such lawsuits. If Wyeth wins, essentially all private, state-court lawsuits over dangerous drugs and medical devices will be wiped out—and a window into the workings of the companies poorly regulated by the FDA will be boarded up.
Indeed, the underlying assumption behind preemption is that federal regulatory agencies actually do a good job of protecting the public, when there is a host of evidence suggesting that’s not the case. In fact, it’s been the very private lawsuits that big companies want to eliminate that have often led to major efforts to strengthen the nation’s health and safety net.
If the Supreme Court follows Troy’s lead, it won’t just be pharmaceutical and medical-device lawsuits that are affected. A ruling for the drug company in Levine could also limit lawsuits over everything from toxic chemical exposure to tainted beef—a pretty grim scenario for the average consumer. And there’s reason to believe the court will do just that.
During the oral arguments in the Kent case last week, Justice Stephen Breyer, one of the high court’s most liberal justices, showed little sympathy for the plaintiffs or confidence in juries. “Who would you rather have make this decision as to whether this drug is, on balance, going to save people or, on balance, going to hurt people?” he asked. “An expert agency, on the one hand, or 12 people pulled randomly for a jury role who see before them only the people whom the drug hurt and don’t see those who need the drug to cure them?”
The arguments showed how successful Troy and other preemption advocates have been in changing the focus on the legal debate, from providing recourse to those injured by faulty products to protecting the companies that make them. They have launched a legal revolution that is as profound as the consumer protection movement Ralph Nader touched off in the 1960s and ’70s, only the beneficiaries are the big corporations that want to escape the last remaining check on their work: private lawsuits. The Supreme Court’s decision in Riegel and its move to take up the other preemption cases suggests that the revolution is indeed having a transformative effect.
Mr Hinchey introduced an amendment to take $500 000 away from Mr Troy's office and add it to the Drug Evaluation Research budget to counteract false and misleading advertising. The US House passed the amendment unanimously.
A spokesman for the FDA said that it was not the FDA that filed briefs that have been described as defending Pfizer; it was the Department of Justice that did. Moreover, the briefs defended the government's own interests, not Pfizer.
In addition, the payment of $358 000 by Pfizer was not to Mr Troy himself but to his firm.

















Dan Troy is NO friend to consumers. He has long protected the pharmaceutical industry concerning FDA issues and government regulations.
I first ran into him back in early 2000s when he was at FDA intervening in private citizen lawsuits against drug companies like the wrongful death/failure to warn lawsuit I had against Pfizer for my husband's Zoloft induced suicide Dan Troy used his role as FDA Chief Counsel to file amicus curiae “friend of the court” briefs intervening on behalf of the pharmaceutical industry. It seemed whenever drug companies faced civil suits regarding their products' harmful side effects--side effects concealed from prescribing physicians and consumers--this brief was being used. Dan Troy was the chief architect behind the FDA Preemption brief. Many civil lawsuits were tossed out by judges across the country who believed the FDA is the final authority on drug labeling. This effort essentially gave drug companies, such as Pfizer, a free pass when their products caused harm and death.
Wonder how many people know that Pfizer was one of Dan Troy’s previous clients at Wiley, Rein, & Fielding and that in the three years before Troy's appointment as FDA Chief Counsel, Pfizer paid the law firm more than $350K for services provided directly by Troy??
Or that during a December 15, 2003, presentation titled, “The Case for Preemption,” at the annual Drug and Medical Device Litigation conference for in-house counsel and trial attorneys, Dan Troy asked the audience to contact him if they had a case in which the FDA could help. Clearly Troy's "help" was not meant to assist consumers. Fortunately, my talented attorney clandestinely attended Troy's lunchtime presentation long enough to hear Troy declare, “we have to pick our shots so make it sound like a Hollywood pitch."
We worked with NY Rep Maurice Hinchey's office to expose Dan Troy. He eventually left the FDA and the revolving regulator door eventually landed Dan Troy as Glaxo Smith Kline's Global Chief Counsel.
Long before President Bush appointed Troy as Chief Counsel for the FDA, Troy assisted drug companies and drug-company supported trade groups such as the Washington Legal Foundation. Under his unofficial FDA leadership, he helped modify the generic-drug application process, oversaw reform to the Hatch-Waxman Act, and was chief architect and author of the original preemption argument used against private citizens in litigation. I take everything Dan Troy says with a grain of salt, especially when it comes to protecting the public.
Here's a blog post with a little more background on Dan Troy that I wrote back in 2017.
https://fiddaman.blogspot.com/2017/04/guest-post-from-kim-witczak-who-is-dan.html
You can tell a shill article by its sources. This WSJ article gives five. The first is a director from Georgetown who is simply stating the obvious and the quote doesn’t need to be attributed. It’s just padding. The second is “legal and public health experts.” The third is an epidemiologist from John’s Hopkins who gives another throwaway quote, more padding. Fourth is a UPENN “bioethicist” who really doesn’t offer much insight. Finally we are given “industry executives” who are defending the FDA fees they pay to ensure “speedy reviews.” No conflict of interest there.
The three named sources are all employees from universities with known Big Pharma ties.
Why can’t they name these experts and executives? A journalist is only as good as his sources and if he can’t name them, he’s useless at best.
In the end, the article is not very damning anyway. “Kennedy’s going to have a tough time doing his job.” Does the author know anything about Kennedy’s history with NRDC? I don’t think he’s worried or naive about the challenges he faces.